Synergistic Anticancer Effects of Fermented Noni Extract Combined with 5-Fluorouracil, Doxorubicin, and Vincristine on A549, MCF-7, and SH-SY5Y Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Sample Preparation
2.3. Cell Culture
2.4. Cell Viability and Synergistic Effects of A549, MCF-7, and SH-SY5Y Cells with XTT Assays and Combination Index
2.5. Cell Migration
2.6. Colony Formation
2.7. Analysis of Apoptosis-Related Protein Expression Using Western Blotting
2.8. Statistical Analyses
3. Results
3.1. Cell Viability and Synergistic Effects of A549, MCF-7, and SH-SY5Y Cells with XTT Assays and Combination Index
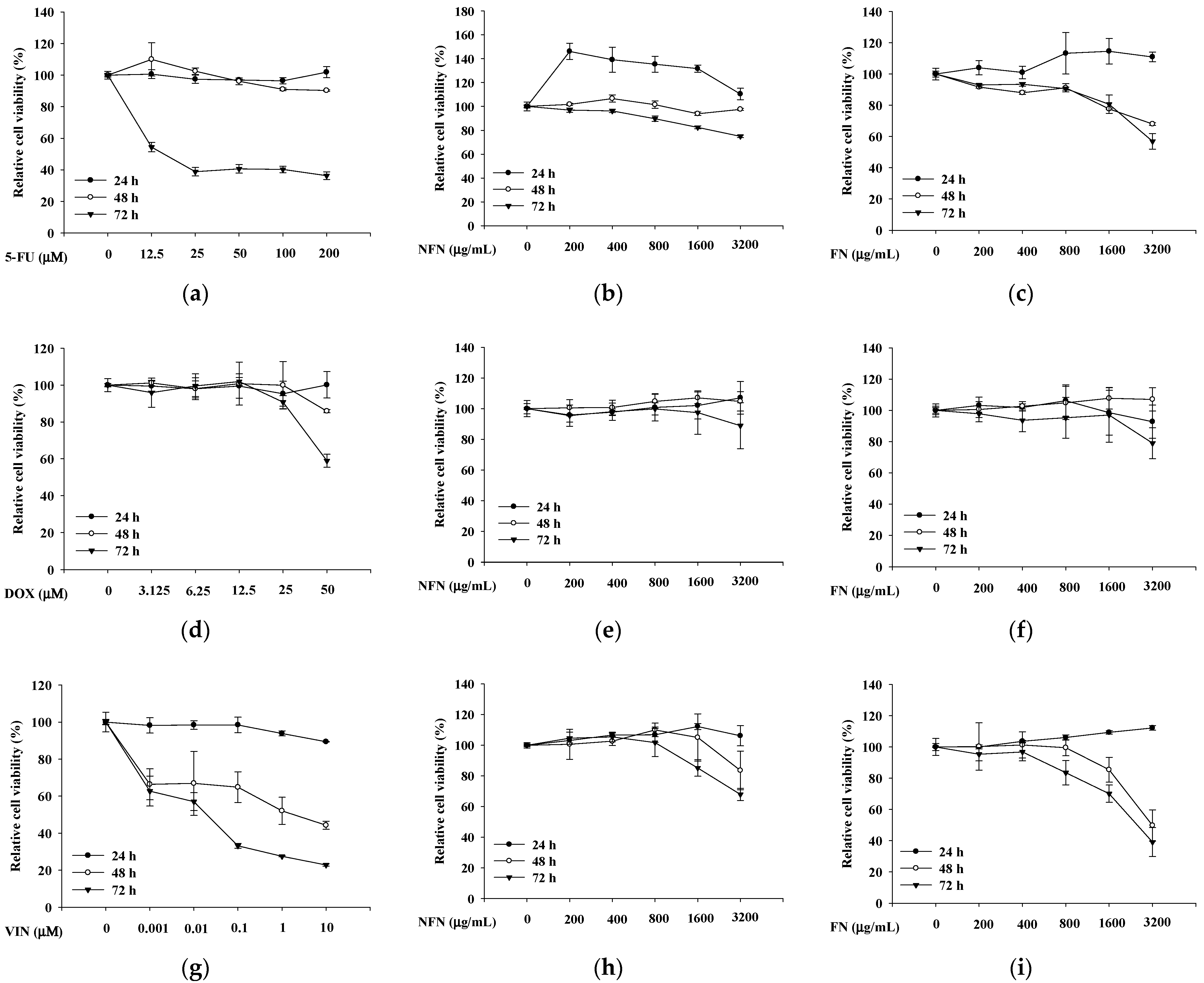
3.1.1. Anticancer Effects of 5-FU, DOX, VIN, NFN, and FN in A549, MCF-7, and SH-SY5Y Cells
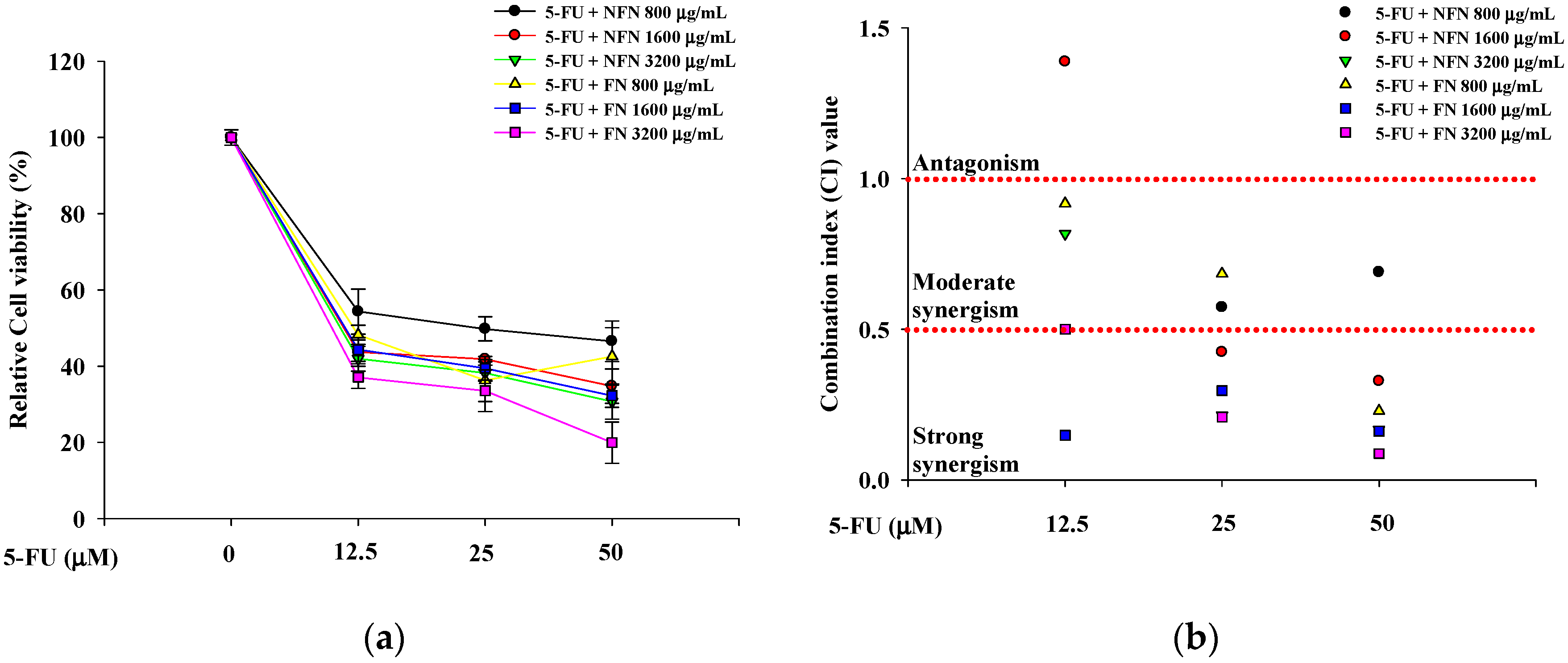
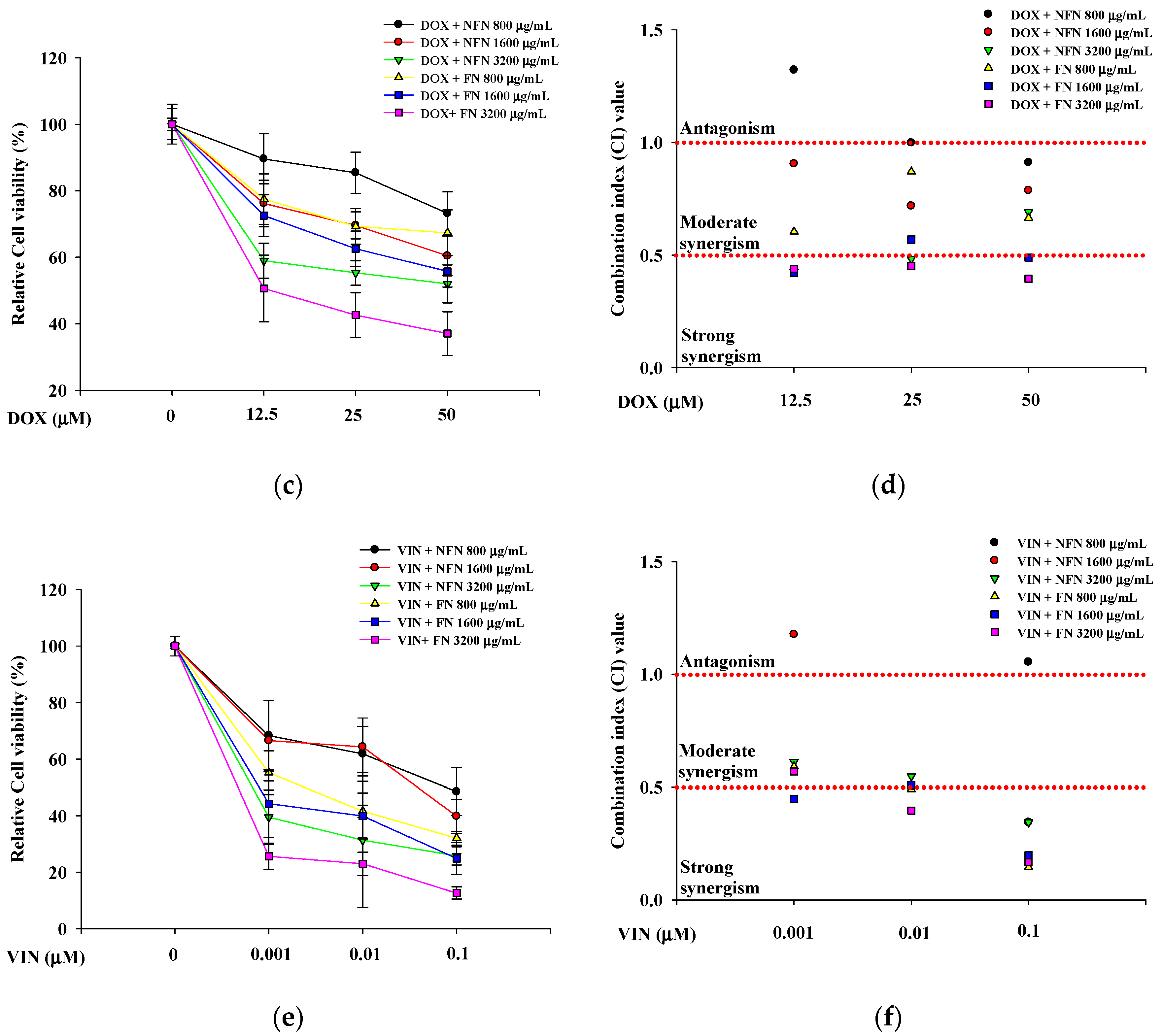
3.1.2. Synergistic Anticancer Effects of 5-FU, DOX, and VIN Combined with NFN or FN in A549, MCF-7, and SH-SY5Y Cells
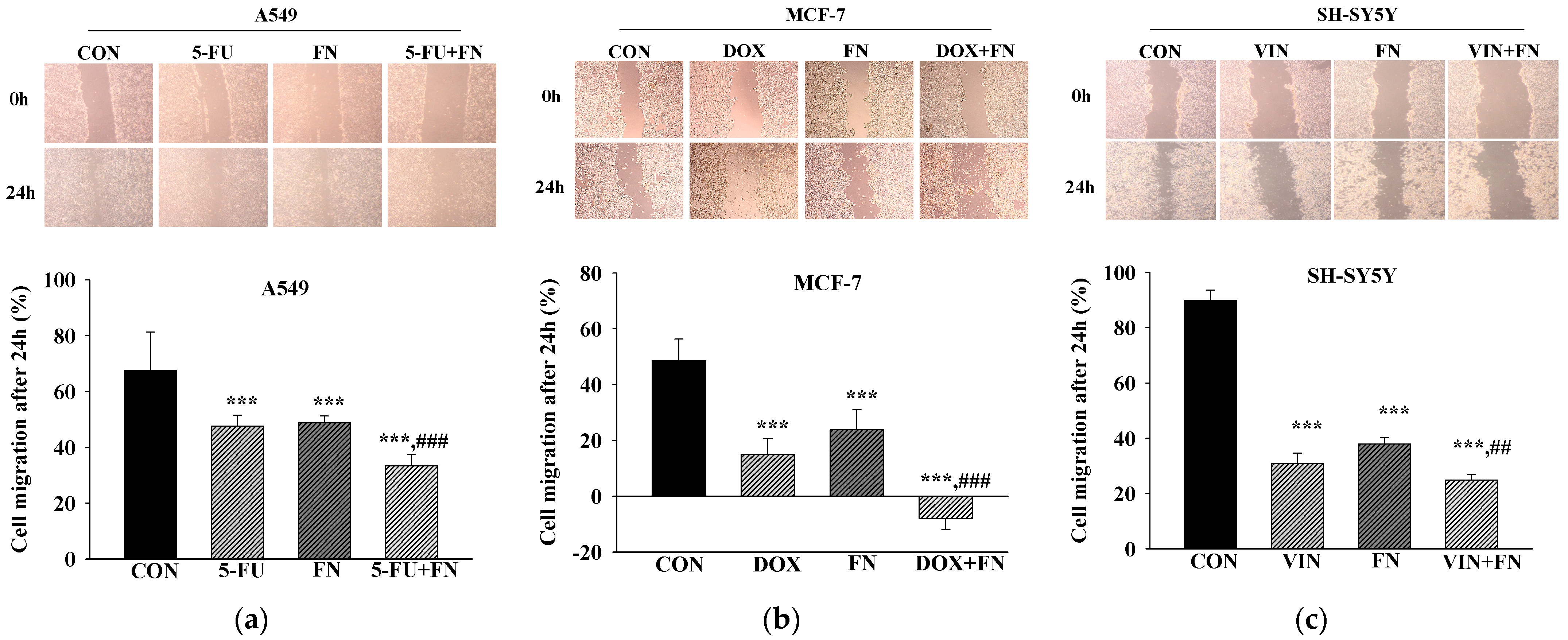
3.2. Effects of 5-FU, DOX, and VIN Combined with NFN or FN on Cell Migration in A549, MCF-7, and SH-SY5Y Cells
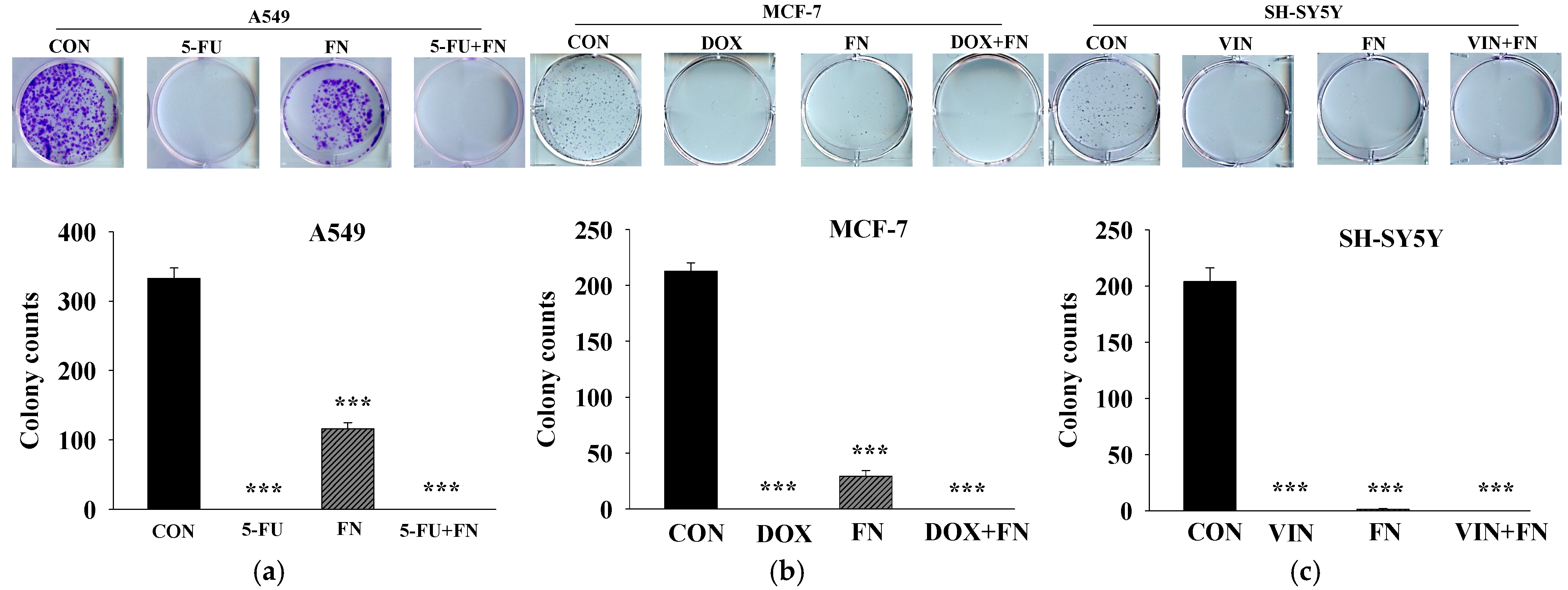
3.3. Effects of 5-FU, DOX, and VIN Combined with NFN or FN on Colony Formation in A549, MCF-7, and SH-SY5Y Cells
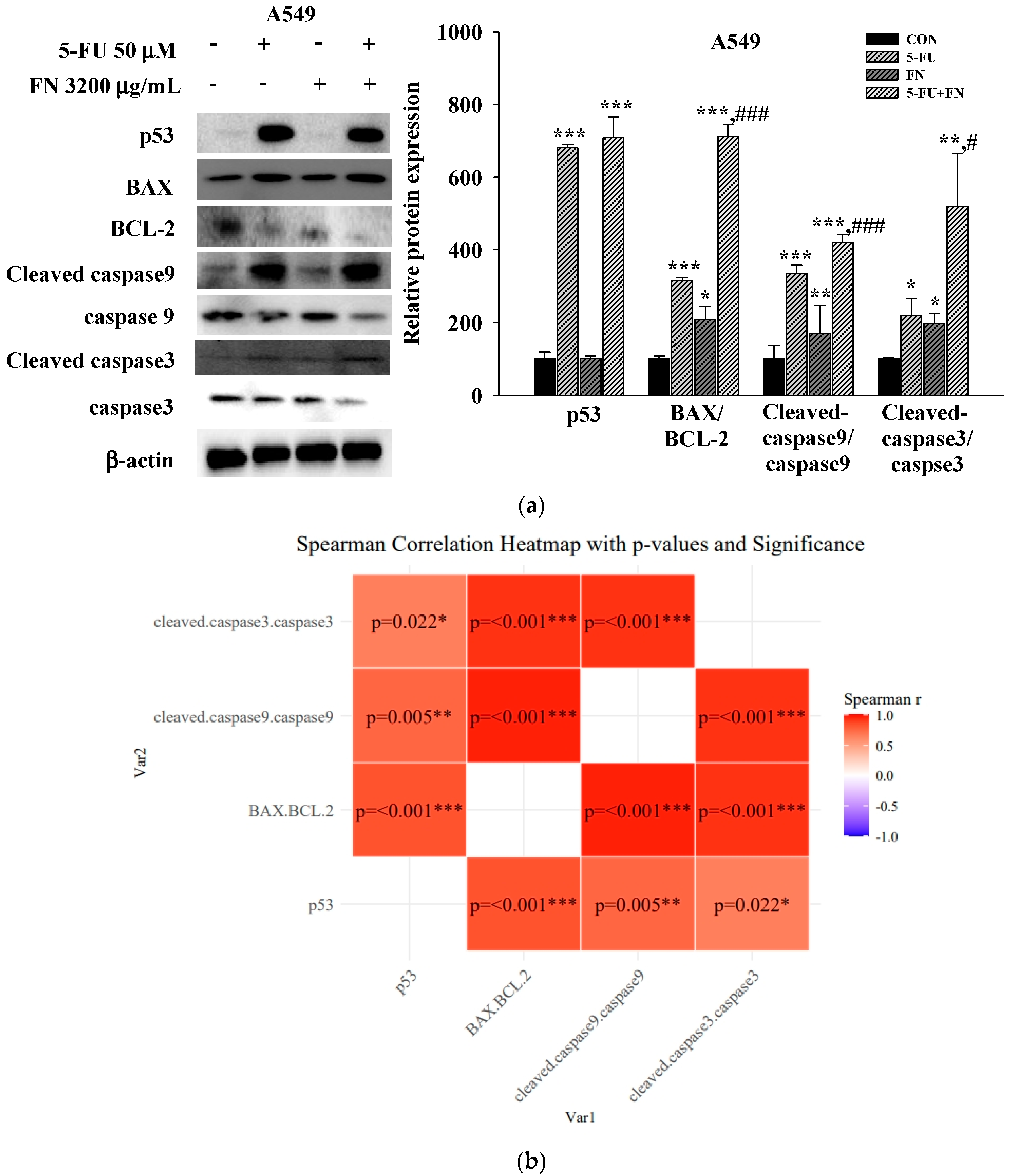
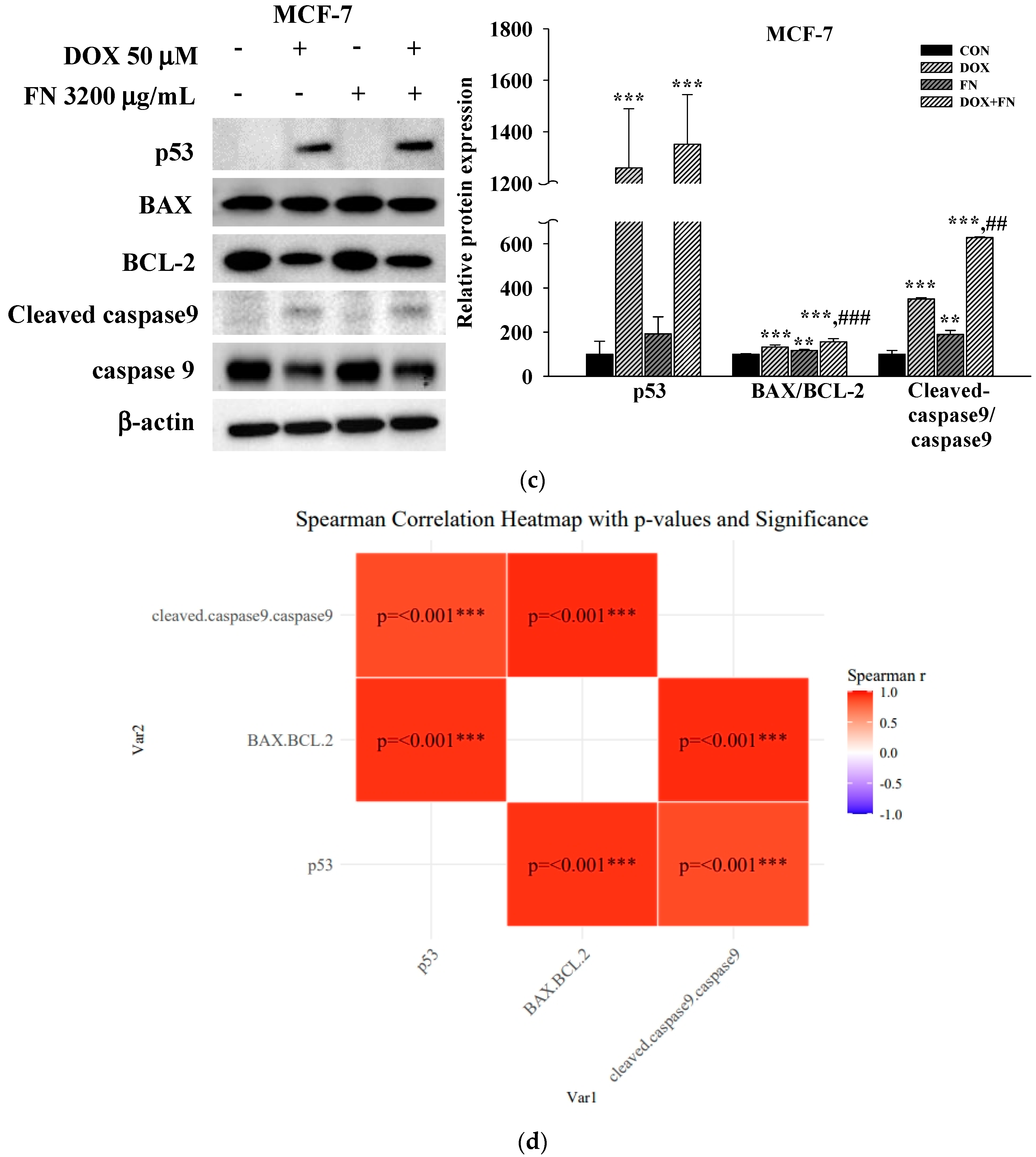
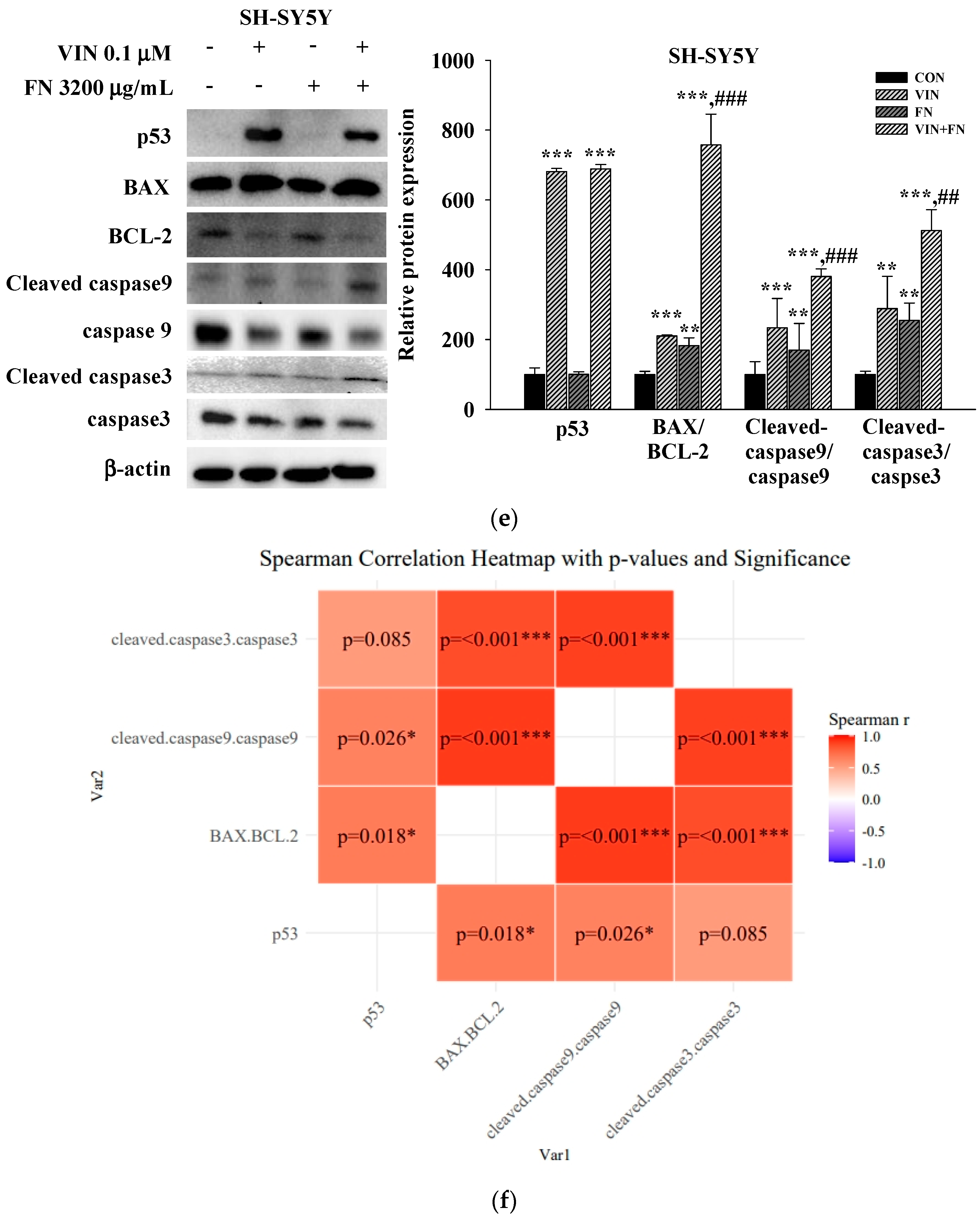
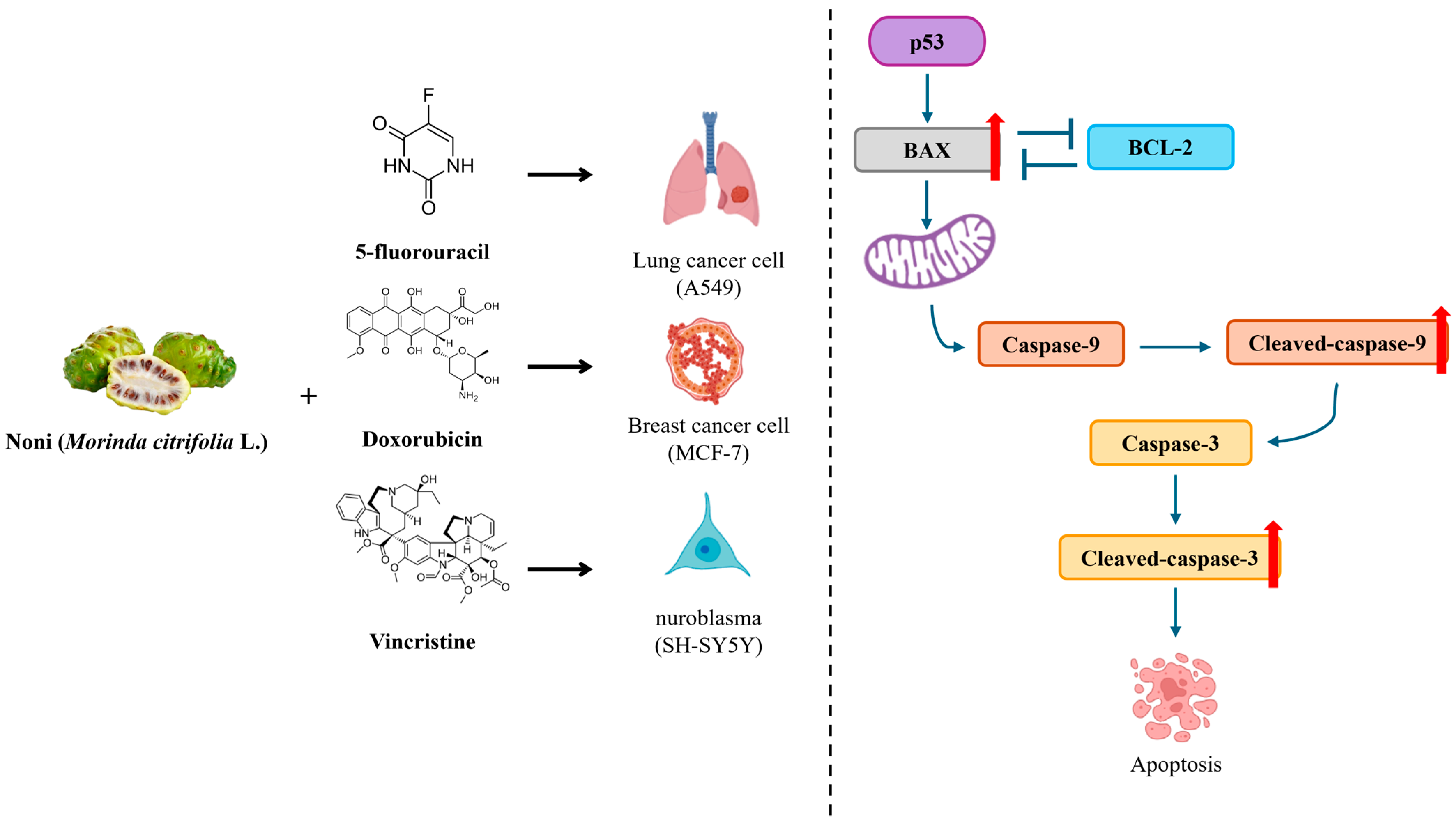
3.4. Effects of 5-FU, DOX, and VIN Combined with NFN or FN on Apoptosis-Related Signaling Pathways in A549, MCF-7, and SH-SY5Y Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potterat, O.; Hamburger, M. Morinda citrifolia (Noni) fruit-phytochemistry, pharmacology, safety. Planta Medica 2007, 73, 191–199. [Google Scholar] [CrossRef]
- Pratap, U.P.; Hima, L.; Priyanka, H.P.; ThyagaRajan, S. Noni (Morinda citrifolia L.) fruit juice reverses age-related decline in neural-immune interactions in the spleens of old F344 rats. J. Ethnopharmacol. 2017, 198, 363–371. [Google Scholar] [CrossRef]
- Dixon, A.R.; McMillen, H.; Etkin, N.L. Ferment this: The transformation of Noni, a traditional Polynesian medicine (Morinda citrifolia, Rubiaceae). Econ. Bot. 1999, 53, 51–68. [Google Scholar] [CrossRef]
- McClatchey, W. From Polynesian healers to health food stores: Changing perspectives of Morinda citrifolia (Rubiaceae). Integr. Cancer Ther. 2002, 1, 110–120. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Zhang, J.; Kilmartin, P.A.; Quek, S.Y. Exploring the effects of microencapsulation on odour retention of fermented noni juice. J. Food Eng. 2020, 273, 109892. [Google Scholar] [CrossRef]
- Motshakeri, M.; Ghazali, H.M. Nutritional, phytochemical and commercial quality of noni fruit: A multi-beneficial gift from nature. Trends Food Sci. Technol. 2015, 45, 118–129. [Google Scholar] [CrossRef]
- Tailulu, A.; Li, M.; Ye, B.; Al-Qudaimi, R.; Cao, F.; Liu, W.; Shi, P. Antimicrobial and anticancer activities of Hainan dry noni fruit alcoholic extracts and their novel compounds identification using UPLC-Q-Exactive Orbitrap-MS/MS. J. Pharm. Biomed. Anal. 2022, 220, 114989. [Google Scholar] [CrossRef]
- Sina, H.; Dramane, G.; Tchekounou, P.; Assogba, M.F.; Chabi-Sika, K.; Boya, B.; Socohou, A.; Adjanohoun, A.; Baba-Moussa, L. Phytochemical composition and in vitro biological activities of Morinda citrifolia fruit juice. Saudi J. Biol. Sci. 2021, 28, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Pachauri, S.D.; Khandelwal, K.; Ahmad, H.; Arya, A.; Biala, P.; Agrawal, S.; Pandey, R.R.; Srivastava, A.; Srivastav, A.; et al. Anticancer effects of extracts from the fruit of Morinda citrifolia (noni) in breast cancer cell lines. Drug Res. 2016, 66, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hirazumi, A.; Furusawa, E. An immunomodulatory polysaccharide-rich substance from the fruit juice of Morinda citrifolia (noni) with antitumour activity. Phytother. Res. 1999, 13, 380–387. [Google Scholar] [CrossRef]
- Prompipak, J.; Senawong, T.; Sripa, T.; Ketterman, A.J.; Utaiwat, S.; Woranam, K.; Jeeunngoi, J.; Senawong, G. Anticancer effects of the combined Thai noni juice ethanolic extracts and 5-fluorouracil against cholangiocarcinoma cells in vitro and in vivo. Sci. Rep. 2021, 11, 14866. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Jensen, C.J.; Westendorf, J.; White, L.D. A safety review of noni fruit juice. J Food Sci. 2006, 71, 100–106. [Google Scholar] [CrossRef]
- Doroshow, J.H.; Simon, R.M. On the design of combination cancer therapy. Cell 2017, 171, 1476–1478. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Buechner-Steudel, P.; Moehler, M.; Schmalenberg, H.; Behrens, R.; Fahlke, J.; Wein, A.; Behl, S.; Kuss, O.; Kleber, G.; et al. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: Two parallel, multicentre phase-II trials. Br. J. Cancer 2009, 101, 1846–1852. [Google Scholar] [CrossRef]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Li, J.; Li, R.; Wu, X.; Gao, F.; Zou, L.; Mak, W.W.S.; Fu, C.; Zhang, J.; et al. Synergistic breast cancer suppression efficacy of doxorubicin by combination with glycyrrhetinic acid as an angiogenesis inhibitor. Phytomedicine 2021, 81, 153408. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Chou, T.C. Preclinical versus clinical drug combination studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Q.; Lei, Z.; Feng, M.; Zhao, Z.; Wang, Y.; Wei, G. DTL promotes cancer progression by PDCD4 ubiquitin-dependent degradation. J. Exp. Clin. Cancer Res. 2019, 38, 350. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, Y.; Chen, Y. Predicting drug combination index and simulating the network-regulation dynamics by mathematical modeling of drug-targeted EGFR-ERK signaling pathway. Sci. Rep. 2017, 7, 40752. [Google Scholar] [CrossRef]
- Polymenis, M. Proteins associated with the doubling time of the NCI-60 cancer cell lines. Cell Div. 2017, 12, 6. [Google Scholar] [CrossRef]
- Wang, S.; He, M.; Li, L.; Liang, Z.; Zou, Z.; Tao, A. Cell-in-cell death is not restricted by caspase-3 deficiency in MCF-7 cells. J. Breast Cancer 2016, 19, 231–241. [Google Scholar] [CrossRef]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Randjelovic, M.; Szopa, A.; Seidel, V.; Prasher, P.; Sharma, M.; Fatima, R.; Ateşşahin, D.A.; et al. Anticancer and chemopreventive potential of Morinda citrifolia L. bioactive compounds: A comprehensive update. Phytother. Res. 2024, 38, 1932–1950. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H.; Zhou, J.; Nishigaki, T.; Li, W.; Liu, T.; Wu, L.; Gao, M. Morinda citrifolia (noni) fruit juice promotes vascular endothelium function in hypertension via the glucagon-like peptide-1 receptor–CaMKKβ–AMPK–eNOS pathway. Phytother. Res. 2020, 34, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bode, A.; Ma, W.Y.; Sang, S.; Ho, C.T.; Dong, Z. Two novel glycosides from the fruits of Morinda citrifolia (noni) inhibit AP-1 transactivation and cell transformation in the mouse epidermal JB6 cell line. Cancer Res. 2001, 61, 5749–5756. [Google Scholar]
- Tian, Q.; Wang, L.; Sun, X.; Zeng, F.; Pan, Q.; Xue, M. Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion, and PI3K/AKT signalling pathway. JBUON 2019, 24, 997–1002. [Google Scholar] [PubMed]
- Labh, A.K.; Priya, V.V.; Gayathri, R. Cytotoxic action of rutin isolated from Morinda citrifolia against hepatic carcinoma cell lines. Drug Invent. Today 2019, 12, 1904–1907. [Google Scholar]
- Huang, C.; Wei, Y.X.; Shen, M.C.; Tu, Y.H.; Wang, C.C.; Huang, H.C. Chrysin, abundant in Morinda citrifolia fruit water–EtOAc extracts, combined with apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. J. Agric. Food Chem. 2016, 64, 4235–4245. [Google Scholar] [CrossRef]
- Zhang, H.; Hassan, Y.I.; Liu, R.; Mats, L.; Yang, C.; Liu, C.; Tsao, R. Molecular mechanisms underlying the absorption of aglycone and glycosidic flavonoids in a Caco-2 cell model. ACS Omega 2020, 5, 26591–26600. [Google Scholar] [CrossRef]
- Kytidou, K.; Bezirtzoglou, E.; Sgouras, D.; Tsiantas, K.; Charalampopoulos, D.; Papanikolaou, S. Plant glycosides and glycosidases: A treasure-trove for bioactivity. Front. Plant Sci. 2020, 11, 357. [Google Scholar] [CrossRef]
- Aziz, M.Y.A.; Omar, A.R.; Subramani, T.; Yeap, S.K.; Ho, W.Y.; Ismail, N.H.; Ahmad, S.; Alitheen, N.B. Damnacanthal is a potent inducer of apoptosis with anticancer activity by stimulating p53 and p21 genes in MCF-7 breast cancer cells. Oncol. Lett. 2014, 7, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, P.; Zhao, Z.; Ding, X.; Xie, F.; Wang, Y.; Li, C. Antitumorigenic effect of damnacanthal on melanoma cell viability through p53 and NF-κB/caspase-3 signaling pathways. Oncol. Lett. 2018, 16, 6039–6044. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.R.; Banerjee, A.; Pathak, S.; Sharma, C.; Singh, N. Induction of mitochondrial-mediated apoptosis by Morinda citrifolia (noni) in human cervical cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 237–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soni, N.R. Novel treatment of cancer and its potential in obesity: “Xeronine” found in Morinda citrifolia (noni fruit). J. Nutr. Disord. Ther. 2018, 8, 1000e135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-S.; Im, J.-H.; Fu, X.; Kim, M.-H.; Seong, Y.-S.; Lee, J.-Y.; Park, E.Y.; Lee, D.s.; La, I.-J.; Lee, O.-H. Synergistic Anticancer Effects of Fermented Noni Extract Combined with 5-Fluorouracil, Doxorubicin, and Vincristine on A549, MCF-7, and SH-SY5Y Cell Models. Curr. Issues Mol. Biol. 2025, 47, 993. https://doi.org/10.3390/cimb47120993
Lim J-S, Im J-H, Fu X, Kim M-H, Seong Y-S, Lee J-Y, Park EY, Lee Ds, La I-J, Lee O-H. Synergistic Anticancer Effects of Fermented Noni Extract Combined with 5-Fluorouracil, Doxorubicin, and Vincristine on A549, MCF-7, and SH-SY5Y Cell Models. Current Issues in Molecular Biology. 2025; 47(12):993. https://doi.org/10.3390/cimb47120993
Chicago/Turabian StyleLim, June-Seok, Ji-Hyun Im, Xiaolu Fu, Min-Hye Kim, Yeon-Seok Seong, Jae-Yeon Lee, Eun Young Park, Do sang Lee, Im-Joung La, and Ok-Hwan Lee. 2025. "Synergistic Anticancer Effects of Fermented Noni Extract Combined with 5-Fluorouracil, Doxorubicin, and Vincristine on A549, MCF-7, and SH-SY5Y Cell Models" Current Issues in Molecular Biology 47, no. 12: 993. https://doi.org/10.3390/cimb47120993
APA StyleLim, J.-S., Im, J.-H., Fu, X., Kim, M.-H., Seong, Y.-S., Lee, J.-Y., Park, E. Y., Lee, D. s., La, I.-J., & Lee, O.-H. (2025). Synergistic Anticancer Effects of Fermented Noni Extract Combined with 5-Fluorouracil, Doxorubicin, and Vincristine on A549, MCF-7, and SH-SY5Y Cell Models. Current Issues in Molecular Biology, 47(12), 993. https://doi.org/10.3390/cimb47120993







