Effects of Exogenous Spermidine on Germination and Seedling Growth of Rice Under Salt Stress: Physiological and Transcriptomic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Design
- (1)
- Germination assay: Germination was assessed according to GB/T 3543.4-1995 [31]. Each germination box (12 cm × 12 cm) was lined with two sheets of filter paper and filled with 10 mL of 75 mM NaCl solution. Pretreated seeds were placed in the boxes, 50 per box, ensuring no contact between seeds. Each treatment was replicated three times. Moisture was maintained by adding NaCl solution as needed. Germination was monitored daily, and measurements were taken 14 days after sowing.
- (2)
- Young plant assay: Pretreated seeds were first grown in seedling trays (60 cm × 30 cm × 3 cm) to the three-leaf stage, then transferred to a hydroponic system containing half-strength Hoagland solution with 75 mM NaCl. Plants were set in hydroponic boxes (125 cm × 85 cm × 115 cm, 6 mm holes) with five plants per hole, secured by cotton plugs. Each treatment had three biological replicates. After 10 days of salt stress, seedlings were collected and stored at −80 °C for physiological and biochemical analysis.
2.3. Measurement Items and Methods
2.3.1. Determination of Relevant Indicators in Rice Germination Tests
2.3.2. Measurement of Relevant Indicators in Rice Seedling Experiments
2.4. Transcriptome Sequencing and Data Analysis
2.5. Statistical Analysis
- (1)
- Data reduction: PCA was applied to extract principal components with cumulative eigenvalues explaining more than 85% of the variance.
- (2)
- Data standardization: Based on the principles of fuzzy mathematics, membership functions were used to normalize the scores of each principal component. The membership function formulas were as follows:
- (3)
- Comprehensive evaluation: The weight (Wj) of each principal component was determined using its cumulative variance contribution rate. The D value was then calculated using the following formula:
3. Results
3.1. Effects of Exogenous Spermidine on Rice Seed Viability Under Salt Stress
3.2. Effects of Exogenous Spermidine on Rice Germination Morphology Under Salt Stress
3.3. Effects of Exogenous Spermidine on the Agronomic Traits of Rice Young Plants Under Salt Stress
3.4. Effects of Exogenous Spermidine on the Physiological and Biochemical Characteristics of Rice Young Plants Under Salt Stress
3.4.1. Effects of Exogenous Spermidine on the Antioxidant Enzyme System of Rice Young Plants Under Salt Stress
3.4.2. Effects of Exogenous Spermidine on Osmotic Regulatory Substances in Rice Young Plants Under Salt Stress
3.4.3. Effects of Exogenous Spermidine on Oxidative Stress in Rice Young Plants Under Salt Stress
3.5. Comprehensive Analysis of the Effects of Exogenous Spermidine on the Growth and Physiology of Rice Young Plants Under Salt Stress
3.5.1. Principal Component Analysis
3.5.2. Subordinate Function Analysis
3.6. Transcriptome Analysis of the Effects of Exogenous Spermidine on the Growth of Rice Young Plants Under Salt Stress
3.6.1. Differential Gene Expression Analysis and Enrichment Analysis
3.6.2. Differential Gene Expression Patterns
3.6.3. qRT-PCR Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | control (no NaCl, no spermidine) |
| S0–S5 | 75 mM NaCl with 0, 0.6, 0.8, 1.0, 1.2, and 1.4 mM spermidine, respectively |
| Spd | Spermidine |
| EBR | Brassinolide |
| KT | Kinetin |
| MT | Melatonin |
| SFW | Shoot Fresh Weight |
| SDW | Shoot Dry Weight |
| RFW | Root Fresh Weight |
| RDW | Root Dry Weight |
| SP | Soluble Sugar |
| SS | Soluble Protein |
| O2−PR | O2− Production Rate |
Appendix A
| Gene_ID | Gene Name | Sequences (5′-3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| Os04g0401000 | PI21 | CTGCGATGCCAAGATCAGGAA | CGTACTCCACCTTCTCGATGC |
| Os03g0760000 | CYP450 | GGGCAACCTGTGCGACTACC | TGTTCCTCACGCCGAACACG |
| Os02g0626100 | OsPAL1 | GAACGGCAAGCCGGAGTACA | CACCTTCTTGGCGTGGCTCAT |
| Os03g0246500 | OsPAL | GACGGAAGCCCTTTCCTTTC | CGAAGAACCGACTGAAACCC |
| Os08g0489300 | Tag I | GTGGCGAAGATGGACGAGAA | GTATCCGCTGAACGACCCAA |
| Os02g0571300 | OsKS5 | GGAGGAATGCGGGAAGCCAA | CTGCACTGGCACCATGGCTA |
| Os09g0127300 | OsCCR17 | TCCTCCATCGGCACTGTCTA | ATTCGAGGTCACTCCAGCAG |
| Os07g0603300 | GL7 | CCCTTGCTGCTCTCCAAATC | ACAGCATTAGCAGGGACCTT |
| LOC4333919 | OsActin | AGGAAGGCTGGAAGAGGACC | CGGGAAATTGTGAGGGACAT |
| Samples | Raw Reads | Clean Reads | N/% | Q30/% | GC/% |
|---|---|---|---|---|---|
| S0-1 | 48247616 | 47612942 | 0.033 | 96.30 | 49.25 |
| S0-2 | 40536848 | 40042426 | 0.032 | 96.41 | 49.54 |
| S0-3 | 44959328 | 44349634 | 0.032 | 96.38 | 49.39 |
| S1-1 | 40839448 | 40361306 | 0.032 | 96.29 | 49.45 |
| S1-2 | 51109362 | 50521482 | 0.033 | 96.44 | 49.44 |
| S1-3 | 36620858 | 36150452 | 0.032 | 96.28 | 49.70 |
| S4-1 | 37261728 | 36731986 | 0.032 | 96.13 | 50.47 |
| S4-2 | 40284046 | 39852256 | 0.033 | 96.42 | 49.83 |
| S4-3 | 44847100 | 44299712 | 0.033 | 96.30 | 49.77 |
References
- Ma, Y.; Tashpolat, N. Current Status and Development Trend of Soil Salinity Monitoring Research in China. Sustainability 2023, 15, 5874. [Google Scholar] [CrossRef]
- Faisal, M.; Faizan, M.; Tonny, S.H.; Rajput, V.D.; Minkina, T.; Alatar, A.A.; Pathirana, R. Strigolactone-Mediated Mitigation of Negative Effects of Salinity Stress in Solanum lycopersicum through Reducing the Oxidative Damage. Sustainability 2023, 15, 5805. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151–161. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhu, X.; Huang, S.; Linquist, B.; Kuzyakov, Y.; Wassmann, R.; Minamikawa, K.; Martinez-Eixarch, M.; Yan, X.; Zhou, F. Greenhouse gas emissions and mitigation in rice agriculture. Nat. Rev. Earth Environ. 2023, 4, 716–732. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Li, T.; Lu, X.; Hu, Q.; Qin, Z. GloRice, a global rice database (v1.0): I. Gridded paddy rice annual distribution from 1961 to 2021. Sci. Data 2025, 12, 182. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, W.; Xu, Y.; Fei, H.; Zhu, Y.; Ahmad, I.; Nimir, N.E.A.; Zhou, G.; Zhu, G. Effects of Salt Stress on Physiological and Agronomic Traits of Rice Genotypes with Contrasting Salt Tolerance. Plants 2024, 13, 1157. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D.; Sujatha, S. Biological and physiological perspectives of specificity in abiotic salt stress response from various rice plants. Asian J. Agric. Sci. 2010, 2, 99–105. [Google Scholar]
- Li, Q.; Zhu, P.; Yu, X.; Xu, J.; Liu, G. Physiological and molecular mechanisms of rice tolerance to salt and drought stress: Advances and future directions. Int. J. Mol. Sci. 2024, 25, 9404. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.-H.; Zhong, C.; Zhu, L.-F.; Cao, X.-C.; Yu, S.-M.; Bohr, J.A.; Hu, J.-J.; Jin, Q.-Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, D.; Zhang, X.; Lv, X.; Li, B. Current progress in deciphering the molecular mechanisms underlying plant salt tolerance. Curr. Opin. Plant Biol. 2025, 83, 102671. [Google Scholar] [CrossRef]
- Li, R.; Guo, X.; Qi, Y.; Wang, Y.; Wang, J.; Zhang, P.; Cheng, S.; He, W.; Zhao, T.; Li, Y.; et al. Soil Amendments and Slow-Release Urea Improved Growth, Physiological Characteristics, and Yield of Salt-Tolerant Rice Under Salt Stress Conditions. Plants 2025, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gao, Q.; Liu, J.; Tang, J.; Hua, Z.; Sun, X. Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur. J. Agron. 2023, 142, 126656. [Google Scholar] [CrossRef]
- Usanmaz, S.; Abak, K. Plant growth and yield of cucumber plants grafted on different commercial and local rootstocks grown under salinity stress. Saudi J. Biol. Sci. 2019, 26, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shi, W. Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: A three-year field experiment. Field Crops Res. 2021, 262, 108027. [Google Scholar] [CrossRef]
- Xiao, M.; Fei, H.; Bu, W.; Zhang, W.; Nimir, N.E.A.; Zhu, G. Research Progress of the Physiological Mechanism of Rice Root Morphology and Physiology Response to Salt Stress and Nitrogen Regulation. J. Anhui Agric. Sci. 2024, 52, 7–10. [Google Scholar]
- de Oliveira, V.P.; Lima, M.D.R.; da Silva, B.R.S.; da Silva Lobato, B.L.B.A.K. Brassinosteroids Confer Tolerance to Salt Stress in Eucalyptus urophylla Plants Enhancing Homeostasis, Antioxidant Metabolism and Leaf Anatomy. J. Plant Growth Regul. 2019, 38, 557–573. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Hasanuzzaman, M. Exogenous kinetin and putrescine synergistically mitigate salt stress in Luffa acutangula by modulating physiology and antioxidant defense. Physiol. Mol. Biol. Plants 2020, 26, 2125–2137. [Google Scholar] [CrossRef]
- Li, J.; Yuan, F.; Liu, Y.; Zhang, M.; Liu, Y.; Zhao, Y.; Wang, B.; Chen, M. Exogenous melatonin enhances salt secretion from salt glands by upregulating the expression of ion transporter and vesicle transport genes in Limonium bicolor. BMC Plant Biol. 2020, 20, 493. [Google Scholar] [CrossRef]
- Al-Mushhin, A.A.M.; Qari, S.H.; Fakhr, M.A.; Alnusairi, G.S.H.; Alnusaire, T.S.; ALrashidi, A.A.; Latef, A.A.H.A.; Ali, O.M.; Khan, A.A.; Soliman, M.H. Exogenous Myo-Inositol Alleviates Salt Stress by Enhancing Antioxidants and Membrane Stability via the Upregulation of Stress Responsive Genes in Chenopodium quinoa L. Plants 2021, 10, 2416. Plants 2021, 10, 2416. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones:An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 2016, 56, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Huang, K.; Batool, M.; Idrees, F.; Afzal, R.; Haroon, M.; Noushahi, H.A.; Wu, W.; Hu, Q.; Lu, X.; et al. Versatile roles of polyamines in improving abiotic stress tolerance of plants. Front. Plant Sci. 2022, 13, 1003155. [Google Scholar] [CrossRef]
- Parrotta, L.; Sobieszczuk-Nowicka, E.; Cai, G. Editorial: Polyamines and longevity—Role of polyamine in plant survival. Front. Plant Sci. 2023, 14, 1232386. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Pang, J.; Meng, F.; Li, Y.; Xu, N.; Yang, C.; Liu, J. Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J. Integr. Agric. 2020, 19, 643–655. [Google Scholar] [CrossRef]
- Korbas, A.; Kubiś, J.; Rybus-Zając, M.; Chadzinikolau, T. Spermidine Modify Antioxidant Activity in Cucumber Exposed to Salinity Stress. Agronomy 2022, 12, 1554. [Google Scholar] [CrossRef]
- Saha, J.; Giri, K.; Roy, S. Identification and characterization of differentially expressed genes in the rice root following exogenous application of spermidine during salt stress. Genomics 2020, 112, 4125–4136. [Google Scholar] [CrossRef]
- Saha, J.; Chaudhuri, D.; Kundu, A.; Bhattacharya, S.; Roy, S.; Giri, K. Phylogenetic, structural, functional characterisation and effect of exogenous spermidine on rice (Oryza sativa) HAK transporters under salt stress. Funct. Plant Biol. 2022, 50, 160–182. [Google Scholar] [CrossRef]
- GB/T 3543.4-1995; Rules for Agricultural Seed Testing—Germination Test. National Standard of the People’s Republic of China S.A.O.: Beijing, China, 1995.
- Vighi, I.L.; Benitez, L.C.; Amaral, M.N.; Moraes, G.P.; Auler, P.A.; Rodrigues, G.S.; Deuner, S.; Maia, L.C.; Braga, E.J.B. Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biol. Plant. 2017, 61, 540–550. [Google Scholar] [CrossRef]
- Zahir, S.; Zhang, F.; Chen, J.; Zhu, S. Determination of Oxidative Stress and Antioxidant Enzyme Activity for Physiological Phenotyping During Heavy Metal Exposure. Methods Mol. Biol. 2021, 2326, 241–249. [Google Scholar]
- Aboutalebiyan, M.A.; Ekbatani, G.Z.; Sephehri, A. Effects of on-farm seed priming with zinc sulfate and urea solutions on emergence properties, yield and yield components of three rainfed wheat cultivars. Ann. Biol. Res. 2012, 3, 4790–4796. [Google Scholar]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, R.L.; Swennen, R. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2006, 347, 201–207. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tang, J.; Zheng, X.; Li, A.; Zhang, J. The regulating mechanism of salt tolerance of black walnut seedlings was revealed by the physiological and biochemical integration analysis. Plant Physiol. Biochem. 2024, 210, 108548. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, M.; Wang, X.; Liu, L.; Wu, H.; Chen, X.; Wang, H.; Shen, Q.; Chen, G.; Wang, Y. Seed-soaking with melatonin for the improvement of seed germination, seedling growth, and the antioxidant defense system under flooding stress. Agronomy 2022, 12, 1918. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, D.; Feng, N.; Khan, A.; Deng, R.; Xiong, J.; Ding, L.; Sun, Z.; Li, J.; Yang, X.; et al. Regulation of Exogenous Strigolactone on Storage Substance Metabolism and Endogenous Hormone Levels in the Early Germination Stage of Rice Seeds Under Salt Stress. Antioxidants 2025, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Gao, Y.; Pandey, B.K.; Peralta Ogorek, L.L.; Zhao, Y.; Quan, R.; Zhao, Z.; Jiang, L.; Huang, R.; et al. The OsEIL1-OsWOX11 transcription factor module controls rice crown root development in response to soil compaction. Plant Cell 2024, 36, 2393–2409. [Google Scholar] [CrossRef]
- Shen, X.; Dai, S.; Chen, M.; Huang, Y. Spermidine augments salt stress resilience in rice roots potentially by enhancing OsbZIP73’s RNA binding capacity. BMC Plant Biol. 2024, 24, 786. [Google Scholar] [CrossRef]
- Zuo, G.; Huo, J.; Yang, X.; Mei, W.; Zhang, R.; Khan, A.; Feng, N.; Zheng, D. Photosynthetic mechanisms underlying NaCl-induced salinity tolerance in rice (Oryza sativa L.). BMC Plant Biol. 2024, 24, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Lavandosque, L.L.; Vischi Winck, F. Polyamine-Mediated Growth Regulation in Microalgae: Integrating Redox Balance and Amino Acids Pathway into Metabolic Engineering. SynBio 2025, 3, 8. [Google Scholar] [CrossRef]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.; Shaygan, A. Foliar application of polyamines improve some morphological and physiological characteristics of rose. Folia Hortic. 2021, 33, 147–156. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Li, W.; Liu, Z.; Tang, S.; Chen, L.; Ding, C.; Jiang, Y.; Ding, Y.; Li, G. Melatonin improves K+ and Na+ homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol. Environ. Saf. 2020, 206, 111358. [Google Scholar] [CrossRef]
- Athar, H.R.; Zafar, Z.U.; Ashraf, M. Glycinebetaine improved photosynthesis in canola under salt stress: Evaluation of chlorophyll fluorescence parameters as potential indicators. J. Agron. Crop Sci. 2015, 201, 428–442. [Google Scholar] [CrossRef]
- Bukhat, S.; Manzoor, H.; Athar, H.; Zafar, Z.U.; Azeem, F.; Rasul, S. Salicylic Acid Induced Photosynthetic Adaptability of Raphanus sativus to Salt Stress is Associated with Antioxidant Capacity. J. Plant Growth Regul. 2020, 39, 809–822. [Google Scholar] [CrossRef]
- Price, J.; Laxmi, A.; Martin, S.K.; Kang, J.C. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 2004, 16, 2128–2150. [Google Scholar] [CrossRef]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-Um, S.; Takabe, T.; Kirdmanee, C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, C.M.; Zou, Z.R. Effects of exogenous spermidine on metabolism of nonstructural carbonhydrate and involved activity of enzymes of tomato seedlings under drought stress. Agric. Res. Arid Areas 2012, 30, 143–148. [Google Scholar]
- Hai, X.; Mi, J.; Zhao, B.; Zhang, B.; Zhao, Z.; Liu, J. Foliar Application of Spermidine Reduced the Negative Effects of Salt Stress on Oat Seedlings. Front. Plant Sci. 2022, 13, 846280. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, L.; Sun, Y.; Zhan, C.; Tang, S.; Qin, T.; Liu, N.; Huang, J. OsNAC120 balances plant growth and drought tolerance by integrating GA and ABA signaling in rice. Plant Commun. 2024, 5, 100782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, B.; Wan, Z.; Chen, X.; Liu, C.; Liu, C.; Zhou, Y. Exogenous Spermidine Promotes Germination of Aged Sorghum Seeds by Mediating Sugar Metabolism. Plants 2022, 11, 2853. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, J.; Zhang, C.; Li, Y.; Gou, H.; Liang, G.; Ma, Z.; Mao, J.; Chen, B. Glucose enhanced lignin accumulation in grapevine stems via promoting phenylpropanoid biosynthesis. Chem. Biol. Technol. Agric. 2024, 11, 152. [Google Scholar] [CrossRef]
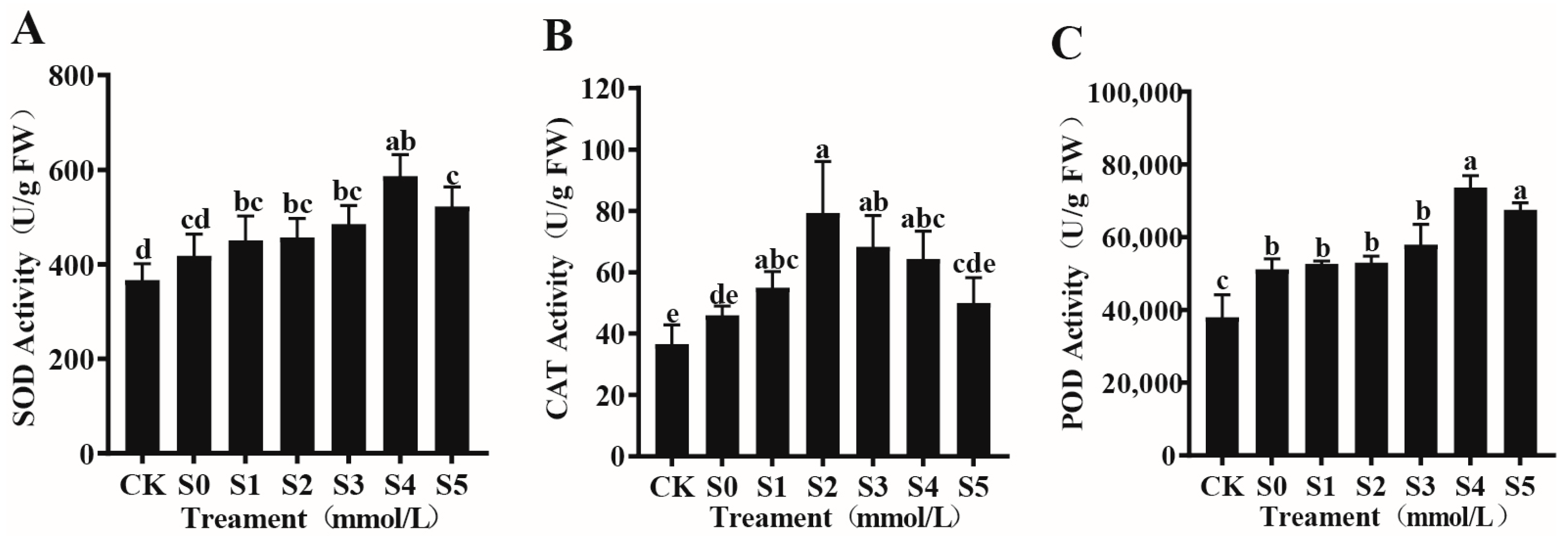
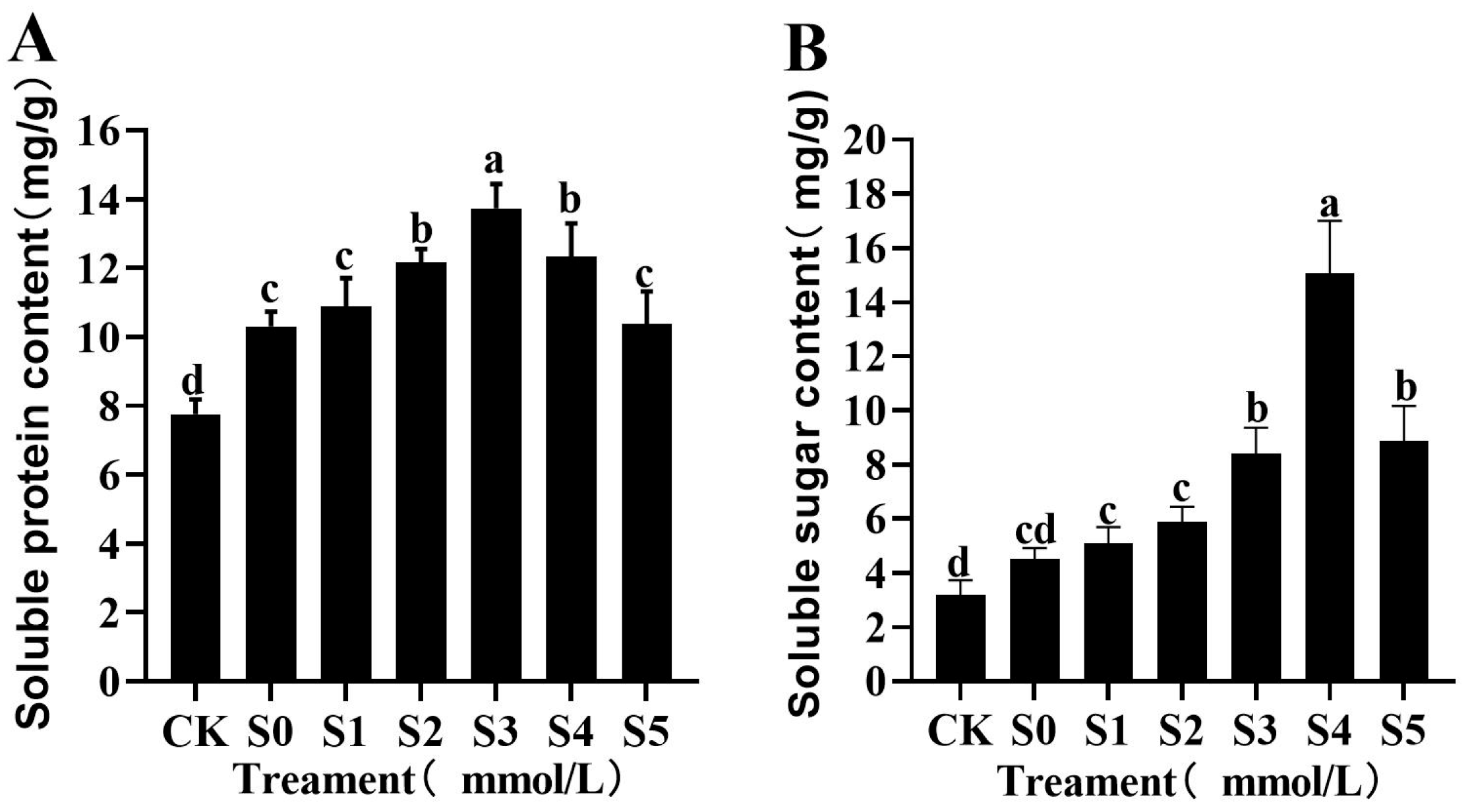
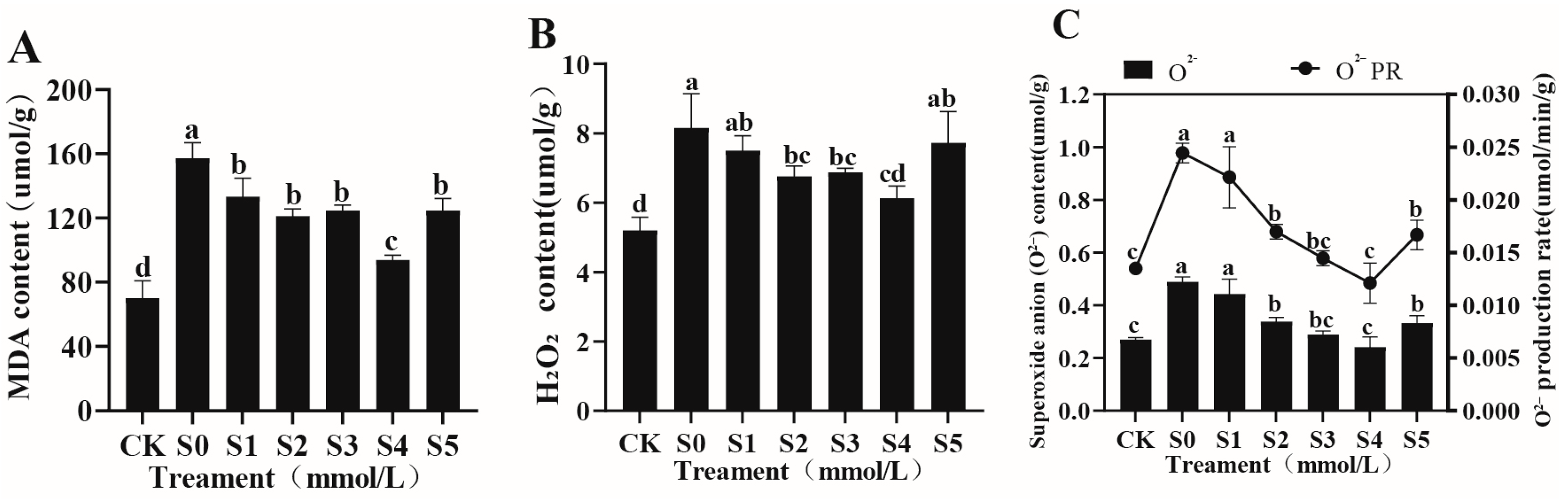
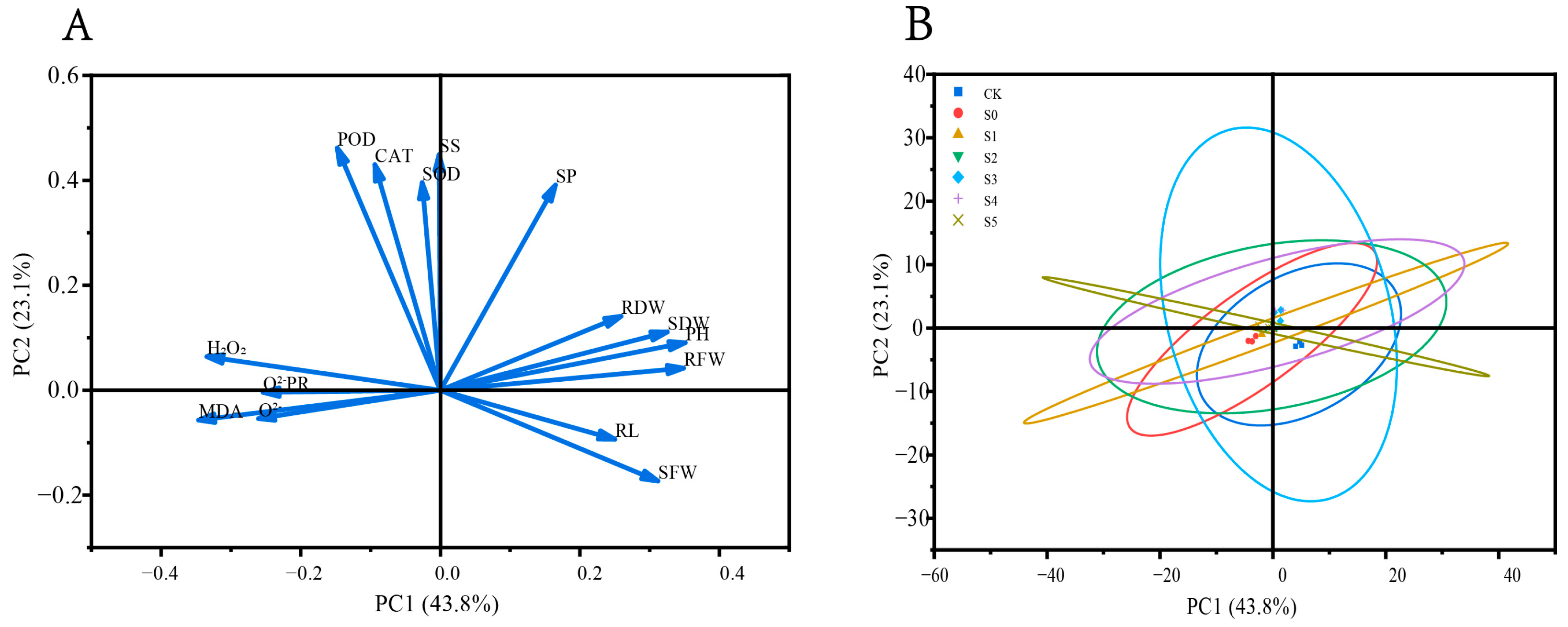
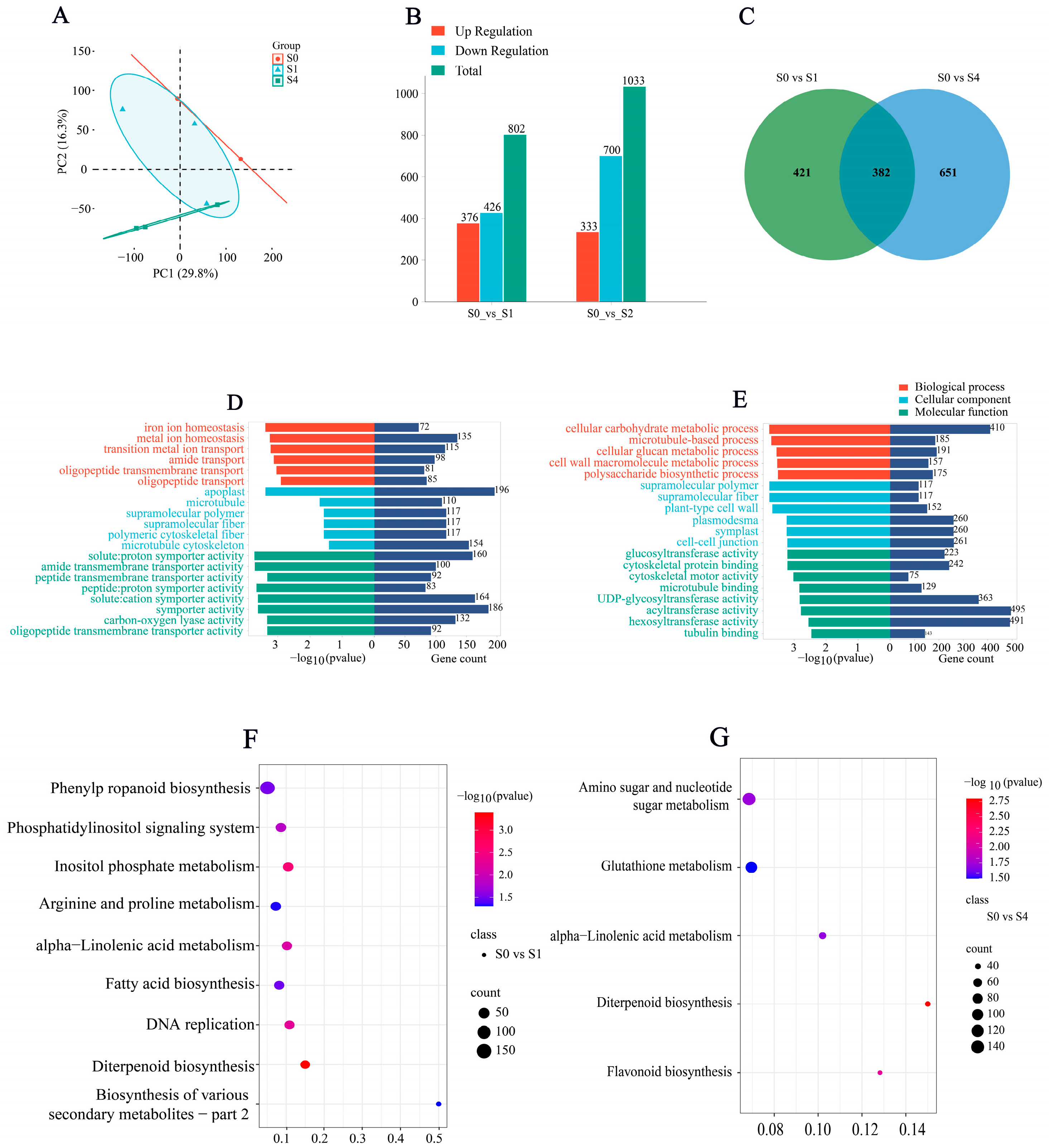
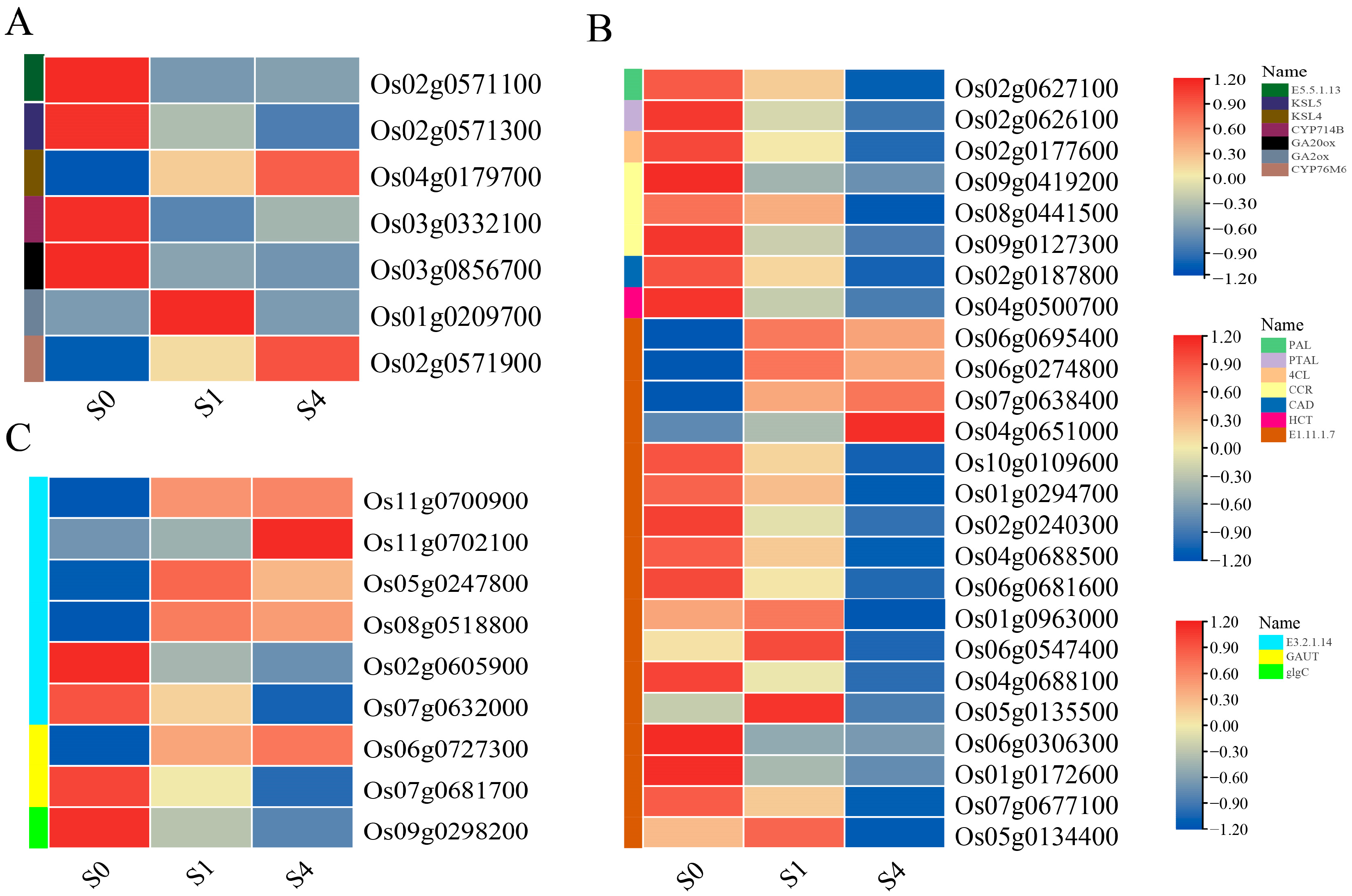
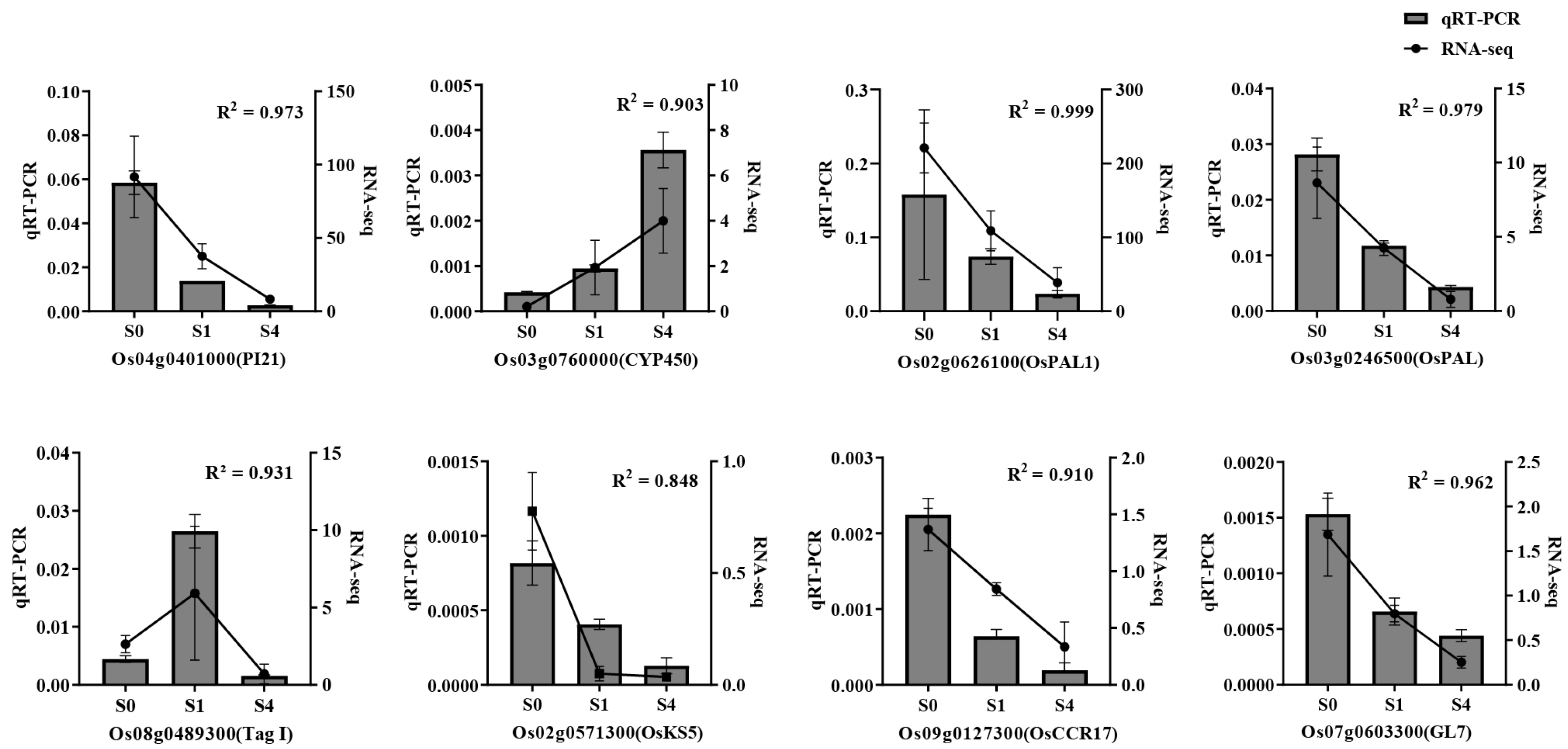
| Treatment | Germination Potential (%) | Germination Rate (%) | Vigor Index | Germination Index |
|---|---|---|---|---|
| CK | 95.50 ± 2.52 a | 96.00 ± 1.63 a | 134.47 ± 9.03 a | 16.66 ± 1.23 a |
| S0 | 88.00 ± 2.00 b | 88.00 ± 2.00 c | 89.05 ± 1.59 d | 12.05 ± 0.23 e |
| S1 | 89.00 ± 2.58 b | 89.50 ± 1.92 bc | 100.45 ± 11.63 bc | 12.62 ± 0.81 de |
| S2 | 89.50 ± 3.79 ab | 92.50 ± 1.00 ab | 107.57 ± 4.64 bc | 13.67 ± 0.55 cd |
| S3 | 91.50 ± 5.26 ab | 94.00 ± 2.83 a | 117.62 ± 9.19 b | 14.62 ± 0.35 bc |
| S4 | 93.00 ± 4.76 ab | 95.50 ± 1.00 a | 130.02 ± 5.46 a | 15.66 ± 0.73 ab |
| S5 | 89.50 ± 3.00 ab | 90.00 ± 4.00 bc | 101.40 ± 6.31 c | 13.15 ± 0.88 de |
| Treatment | Seedling Length (cm) | Seedling Root Length (cm) | Number of Roots | Aboveground Fresh Weight (mg) | Belowground Fresh Weight (mg) | Aboveground Dry Weight (mg) | Belowground Dry Weight (mg) |
|---|---|---|---|---|---|---|---|
| CK | 8.09 ± 0.67 a | 9.01 ± 0.85 a | 6.23 ± 0.79 a | 29.57 ± 2.44 ab | 55.89 ± 2.15 a | 6.68 ± 0.49 ab | 12.88 ± 0.54 a |
| S0 | 7.39 ± 0.10 b | 6.82 ± 0.35 c | 4.73 ± 0.25 c | 25.63 ± 0.65 d | 49.61 ± 0.84 c | 5.93 ± 0.32 b | 11.20 ± 0.46 b |
| S1 | 7.94 ± 0.48 ab | 6.78 ± 0.45 c | 5.65 ± 0.37 ab | 26.88 ± 1.64 bc | 49.56 ± 0.79 c | 6.20 ± 0.95 ab | 11.43 ± 0.87 b |
| S2 | 7.88 ± 0.32 ab | 6.85 ± 0.43 c | 5.45 ±0.24 abc | 29.02 ± 0.93 abc | 51.76 ± 1.11 bc | 7.07 ± 0.15 a | 13.23 ± 1.02 a |
| S3 | 8.04 ± 0.44 ab | 8.03 ± 0.55 b | 5.73 ± 0.56 ab | 29.50 ± 1.69 ab | 52.12 ± 2.22 b | 7.17 ± 0.35 a | 13.23 ± 0.74 a |
| S4 | 8.31 ± 0.23 a | 9.39 ± 0.62 a | 6.03 ± 0.39 ab | 30.21 ± 1.52 a | 56.99 ± 1.20 a | 7.15 ± 0.59 a | 13.75 ± 0.62 a |
| S5 | 7.72 ± 0.20 ab | 7.60 ± 0.89 bc | 5.23 ± 0.52 bc | 27.24 ± 1.04 bcd | 51.84 ± 1.09 bc | 5.90 ± 0.46 b | 12.85 ± 0.48 a |
| Treatment | Plant Height (cm) | Root Length (cm) | Shoot Fresh Weight (mg) | Shoot Dry Weight (mg) | Root Fresh Weight (mg) | Root Dry Weight (mg) |
|---|---|---|---|---|---|---|
| CK | 24.29 ± 2.15 a | 9.96 ± 2.71 ab | 133.63 ± 7.34 a | 84.87 ± 6.92 a | 24.87 ± 3.67 ab | 34.67 ± 2.08 bc |
| S0 | 18.39 ± 1.01 cd | 7.21 ± 0.58 c | 88.24 ± 7.23 c | 59.43 ± 6.50 c | 14.40 ± 2.16 d | 26.33 ± 3.51 d |
| S1 | 20.05 ± 1.39 bc | 7.46 ± 0.76 bc | 105.50 ± 14.55 b | 67.67 ± 8.56 bc | 20.67 ± 0.58 c | 31.33 ± 3.06 c |
| S2 | 21.23 ± 0.33 b | 9.88 ± 0.55 ab | 134.68 ± 5.12 a | 75.87 ± 4.77 ab | 21.53 ± 0.92 bc | 37.67 ± 2.31 b |
| S3 | 21.45 ± 0.49 b | 10.58 ± 1.32 a | 116.23 ± 4.05 b | 76.70 ± 1.66 ab | 22.53 ± 1.29 abc | 43.67 ± 3.51 a |
| S4 | 20.77 ± 0.85 b | 7.60 ± 1.36 bc | 115.77 ± 4.07 b | 82.80 ± 5.72 a | 26.00 ± 2.00 a | 46.33 ± 2.52 a |
| S5 | 17.50 ± 0.77 d | 7.18 ± 0.77 c | 84.87 ± 12.00 c | 68.47 ± 3.35 bc | 16.10 ± 0.85 d | 34.33 ± 1.53 bc |
| Character | Factor Loading | ||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| PH (m) | 0.351 | 0.091 | 0.144 | 0.234 | 0.081 |
| RL (cm) | 0.250 | −0.093 | −0.106 | −0.263 | 0.651 |
| SFW (mg) | 0.312 | −0.173 | 0.298 | −0.129 | 0.167 |
| SDW (mg) | 0.325 | 0.111 | 0.295 | 0.233 | 0.005 |
| RFW (mg) | 0.349 | 0.042 | 0.199 | 0.283 | −0.174 |
| RDW (mg) | 0.259 | 0.141 | 0.091 | −0.139 | −0.556 |
| MDA (μmol/g) | −0.346 | −0.057 | −0.109 | 0.062 | −0.045 |
| POD (U/g FW) | −0.148 | 0.461 | 0.020 | −0.178 | 0.092 |
| CAT (U/g FW) | −0.094 | 0.430 | −0.215 | 0.236 | 0.060 |
| SOD (U/g FW) | −0.026 | 0.396 | −0.093 | 0.544 | 0.185 |
| SP (mg/g) | 0.165 | 0.391 | 0.024 | −0.392 | −0.100 |
| SS (mg/g) | −0.002 | 0.449 | 0.208 | −0.351 | 0.169 |
| H2O2 (umol/g) | −0.334 | 0.064 | 0.301 | −0.084 | −0.185 |
| O2-(umol/g) | −0.261 | −0.054 | 0.584 | 0.000 | 0.018 |
| O2-PR (umol/min/g) | −0.254 | −0.004 | 0.449 | 0.188 | 0.284 |
| Eigenvalue | 6.57 | 3.47 | 1.48 | 0.99 | 0.74 |
| Percentage of Variance (%) | 43.81 | 23.11 | 9.89 | 6.59 | 4.91 |
| Cumulative (%) | 43.81 | 66.92 | 76.82 | 83.41 | 88.32 |
| Treatment | Membership Function | D Value | Rank | ||||
|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |||
| CK | 0.949 | 0.052 | 0.405 | 0.494 | 0.657 | 0.603 | 3 |
| S0 | 0.072 | 0.19 | 0.424 | 0.498 | 0.699 | 0.209 | 7 |
| S1 | 0.333 | 0.362 | 0.252 | 0.665 | 0.528 | 0.367 | 6 |
| S2 | 0.439 | 0.537 | 0.259 | 0.591 | 0.472 | 0.458 | 4 |
| S3 | 0.568 | 0.877 | 0.195 | 0.793 | 0.565 | 0.624 | 2 |
| S4 | 0.54 | 0.958 | 0.535 | 0.3 | 0.777 | 0.644 | 1 |
| S5 | 0.331 | 0.541 | 0.809 | 0.413 | 0.469 | 0.453 | 5 |
| Wj | 0.496 | 0.262 | 0.112 | 0.075 | 0.056 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, B.; Liu, J.; Mao, B.; Wang, R.; Meng, Y.; Huang, H.; Lu, X.; Zhao, F.; Duan, Y. Effects of Exogenous Spermidine on Germination and Seedling Growth of Rice Under Salt Stress: Physiological and Transcriptomic Insights. Curr. Issues Mol. Biol. 2025, 47, 946. https://doi.org/10.3390/cimb47110946
Fei B, Liu J, Mao B, Wang R, Meng Y, Huang H, Lu X, Zhao F, Duan Y. Effects of Exogenous Spermidine on Germination and Seedling Growth of Rice Under Salt Stress: Physiological and Transcriptomic Insights. Current Issues in Molecular Biology. 2025; 47(11):946. https://doi.org/10.3390/cimb47110946
Chicago/Turabian StyleFei, Biaoxin, Jian Liu, Baolai Mao, Ruixiang Wang, Yifan Meng, Haidong Huang, Xin Lu, Fei Zhao, and Yongbo Duan. 2025. "Effects of Exogenous Spermidine on Germination and Seedling Growth of Rice Under Salt Stress: Physiological and Transcriptomic Insights" Current Issues in Molecular Biology 47, no. 11: 946. https://doi.org/10.3390/cimb47110946
APA StyleFei, B., Liu, J., Mao, B., Wang, R., Meng, Y., Huang, H., Lu, X., Zhao, F., & Duan, Y. (2025). Effects of Exogenous Spermidine on Germination and Seedling Growth of Rice Under Salt Stress: Physiological and Transcriptomic Insights. Current Issues in Molecular Biology, 47(11), 946. https://doi.org/10.3390/cimb47110946





