Abstract
Ototoxicity is the drug-induced damage of the inner ear, causing bilateral irreversible sensorineural hearing loss. Cisplatin is a widely used chemotherapeutic agent which causes ototoxicity as its side effect. Pretreatment with metformin prior to the application of cisplatin significantly decreased the late apoptosis and attenuated the cisplatin-induced increase in ROS. To understand the molecular mechanisms that are involved in the preventive effect of metformin, we evaluated the change of gene expression induced by cisplatin at several different time points (0 h, 6 h, 15 h, 24 h and 48 h) and the alteration of gene expression according to pretreatment with metformin in HEI-OC1 cells through microarray analysis. Cisplatin exposure induced a total of 89 DEGs (differentially expressed genes) after 6 h, with a total of 433 DEGs after 15 h, a total of 941 DEGs after 24 h, and a total of 2764 DEGs after 48 h. When cells were pretreated with metformin for 24 h, we identified a total of 105 DEGs after 6 h of cisplatin exposure, a total of 257 DEGs after 15 h, a total of 1450 DEGs after 24 h, and a total of 1463 DEGs after 48 h. The analysis was performed based on the gene expression, network analyses, and qRT-PCR, and we identified several genes (CSF2, FOS, JUN, TNFα, NFκB, Txnip, ASK1, TXN2, ATF3, TP53, IL6, and IGF1) as metformin-related preventive biomarkers in cisplatin ototoxicity.
1. Introduction
Sensorineural hearing loss is irreversible and damaged inner ear hair cells do not regenerate after the loss. In avian, cochlea and vestibular hair cell is reported to regenerate after the exposure to noise or ototoxic drugs. However, the inner ear regeneration is scare in mammals, and it is not until recently that neonatal mouse cochlea harbor cells that are capable of regeneration within a week after birth. Therefore, numerous research regarding inner ear hair cell loss due to age, noise exposure, ototoxicity and so on, has focused on the prevention or the protection of these sensory organ.
Ototoxicity is a drug-induced damage of inner ear causing bilateral progressive irreversible sensorineural hearing loss, tinnitus and imbalance. Cisplatin is widely used chemotherapeutic agents but one of its side effects is ototoxicity with the average incidence of 62–100% [1,2]. Hearing loss has severe impacts on the quality of life. It prevents communication and participation in social networks. It has adverse effects on individual’s cognition, mental health and reported to be related to dementia. Additionally, when it occurs in very young children, it delays speech development and seriously affects cognitive and psychosocial development. Thus, it is important to treat or prevent the hearing loss caused by use of cisplatin. In the research regarding the cisplatin ototoxicity, it is important to identify the protective agents or the mechanism that maintain the drugs’ role as a treatment agent but to reduce their side effects.
Several antioxidants have provided efficacy in reducing cisplatin-induced hearing loss in animal models [3,4]. Drugs containing thiol group such as N-acetyl cysteine, sodium thiosulfate, D-methionine, and lipoic acid have a high affinity for cisplatin and reported to have some protective effects [3,4]. A calcium channel blocking agent, flunarizine, reduced cell death by activation of antioxidant protective mechanisms in the cochlea Nrf2 (Nuclear factor erythroid-2-related factor 2) and heme oxygenase-1 [5], and inhibited inflammatory pathways by reducing the activity of NF-kB (nuclear factor kB) [5,6]. The adenosine A1 receptor agonist R-PIA was reported to have protect effect against cisplatin ototoxicity in the rat by exerting an anti-inflammatory effect by preventing ROS (reactive oxygen species) from being generated by the NOX3 (NADPH oxidase 3) enzyme and by downregulation of the STAT-1 (signal tranducer and activator of transcription 1) inflammatory pathway [7]. The cannabinoid 2 (CB2) receptor agonist protected against cisplatin ototoxicity by inhibition of STAT1, thereby preventing cell death in the cochlea [8]. Intratympanic application of siRNAs against TRPV1, NOX3, and STAT1 provided protection against cisplatin ototoxicity in rat model by decreasing ROS generation and preventing inflammation in the cochlea [3].
There are also several promising agents for protection against cisplatin-induced hearing loss. Clinical studies on sodium thiosulfate [9,10], intratympanic N-acetylcysteine injection, intratympanic dexamethasone injection [11,12,13], amifostine [14,15,16] and vitamin E [17] are on the way, but currently, there are no FDA-approved treatments available. It would be promising if it is possible to identify the inner ear protective effects of pre-existing medications or agents which do not interfere with cisplatin’s tumor-killing efficacy.
Metformin is a conventional therapeutic medication for type II diabetes mellitus to control the glucose level. Other than the drug’s role as an anti-diabetic drug, previous studies reported that metformin has shown to prevent oxidative stress-induced cell death [18], has been considered as a potent anticancer drug and mostly [19], and has gained significant attention as an anti-aging drug [20]. Key signaling pathways involved in senescence focus on AMP-activated protein kinase (AMPK) and mTOR (mammalian target of rapamycin) [21]. AMPK plays a crucial part in tissue energy and in immune response, and the activation of the AMPK signaling pathway inhibits various relevant immune signaling pathways such as NF-κB, JAK/STAT, C/EBPβ, HIF-1α and CHOP pathways. Another important pathway involves mTOR and mTOR is a highly conserved serine/threonine protein kinase, which controls the growth of cells and induced by growth factors and nutrients. Metformin is known to exert direct immunomodulatory effect on immune cells by AMPK induction and mTOR inhibition.
In our previous studies with metformin, pretreatment with metformin prior to the application of cisplatin significantly decreased the frequency of late apoptosis in HEI-OCI cells [22]. Metformin attenuated the cisplatin-induced increase in ROS, inhibited the activation of caspase-3 and levels of poly-ADP-ribose polymerase (PARP) and prevented the cisplatin-induced elevation in intracellular calcium concentrations. However, AMPK and mTOR related pathways which are the main mechanism of metformin were not identified in our previous study.
In this study, we evaluated the change of gene expression induced by cisplatin at several different time points, and the alteration of gene expression according to pretreatment with metformin in HEI-OC1 cells through microarray analysis. We also identified potential molecular modulator genes and pathways to determine the role of metformin in the cisplatin ototoxicity.
2. Material and Methods
2.1. HEI-OC1 Cell Culture
The HEI-OC1 cell line is extremely sensitive to ototoxic drugs, expresses several molecular markers which are characteristic of organ of Corti sensory cells [23], and therefore the HEI-OC1 cell line can be a useful study model of ototoxic drugs. The cells were maintained in high-glucose Dulbecco’s modified eagle’s medium (Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA) without antibiotics at 33 °C and 10% CO2 in air.
2.2. Cell Viability after Exposure to Cisplatin and Metformin
Cell viability was measured using a cell counting kit-8 (Dojindo Laboratories, Kumamoto, Japan). HEI-OC1 cells were seeded in 48-well plates, with 2 × 104 cells in each well. The following day, the cells in metformin group and metformin/cisplatin group were treated with 1 mM of metformin. After 24 h, 15 μM of cisplatin which concentration is known to be result in 50% of cell viability in our experiments, were added to cisplatin group and metformin/cisplatin group. After 48 h, the CCK-8 solution was added to each well, and the plates were incubated for 30 min at 33 °C. The optical density was measured at 450 nm using a microplate reader (Spectra Max plus 384; Molecular devices, Sunnyvale, CA, USA).
2.3. Measurement of Caspase-8, Caspase-9 and Caspase-3 Activity at Different Time Points
To identify the effect of metformin on cisplatin-induced cell death at different time points, we divided cells in to 6 h, 12 h, 24 h, and 48 h groups. The cells in metformin/cisplatin group were treated with 1 mM of metformin. After 24 h, 15 μM of cisplatin were added to cisplatin group and metformin/cisplatin group. Auditory cell line lysates were obtained after 6 h, 12 h, 24 h and 48 h.
The enzymatic activity of caspase-8, -9, and -3 was assayed with the fluorometric assay kit (K112, K118, K105; Biovision, Milpitas, California, USA) according to the manufacturer’s protocol. Auditory cell line lysate was prepared in a lysis buffer on ice for 10 min. After measuring the amount of protein, 50 ug of each sample was used. The sample was reacted with each substrate (LEHD-AFC, IETD-AFC, DEVD-AFC) for 2 h at 37 °C. The plates were read by microplate reader (Spectra Max, Molecular Devices, Sunnyvale, CA, USA) at a 400 nm excitation filter and a 505 nm emission filter. We obtained the results by performing the experiment of 5 times.
2.4. RNA Sample Preparation
To identify the effect of metformin on cisplatin-induced cell death and gene expression changes at different time points. HEI-OC1 cells were treated with 15 μM cisplatin, 1 mM metformin for 6 h, 12 h, 24 h, and 48 h and harvested. Their total RNA was extracted using RNasy plus mini kit (QIAGEN, Germany), according to the manufacturer’s instructions. RNA purity and integrity were evaluated by ND-1000 Spectrophotometer (Wilmington, NC, USA), Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). The RNA samples were used for mRNA microarray, and qRT-PCR experiments.
2.5. mRNA MicroArray Analysis
The Affymetrix Whole transcript Expression array process was executed according to the manufacturer’s protocol (GeneChip Whole Transcript PLUS reagent Kit), Applied Biosystems, Waltham, MA, USA). cDNA was synthesized using the GeneChip WT (Whole Transcript). Amplification kit as described by the manufacturer. The sense cDNA was then fragmented and biotin-labeled with TdT (terminal deoxynucleotidyl transferase) using the GeneChip WT Terminal labeling kit. Approximately 5.5 μg of labeled DNA target was hybridized to the Affymetrix GeneChip Mouse 2.0 ST Array at 45 °C for 16 h. Hybridized arrays were washed and stained on a GeneChip Fluidics Station 450 and scanned on a GCS3000 Scanner (Affymetrix). Signal values were computed using the Affymetrix® GeneChip™ Command Console software.
2.6. Data Analysis
Raw data were extracted automatically in Affymetrix data extraction protocol using the software provided by Affymetrix GeneChip® Command Console® Software (AGCC). After importing CEL files, the data were summarized and normalized with robust multi-average (RMA) method implemented in Affymetrix® Expression Console™ Software (EC). We exported the result with gene level RMA analysis and performed the differentially expressed gene (DEG) analysis.
Statistical significance of the expression data was determined using fold change. We investigated gene groups satisfying a FC ≥ 1.5 and p < 0.05 and used statistical methods to compare gene expression among the groups. For a DEG set, Hierarchical cluster analysis was performed using complete linkage and Euclidean distance as a measure of similarity.
Gene-Enrichment and Functional Annotation analysis for significant probe list was performed using KEGG (www.genome.jp/kegg/ accessed on 23 December 2022) and Gene Ontology (www.geneontology.org/ accessed on 23 December 2022). All data analysis and visualization of differentially expressed genes was conducted using R 3.1.2 (www.r-project.org accessed on 23 December 2022), and Morpheus (https://software.broadinstitute.org/morpheus accessed on 23 December 2022). To facilitate biological interpretation, we performed network analysis among the genes that were significantly expressed oppositely in response to cisplatin and the application of metformin (STRING; https://string-db.org accessed on 23 December 2022).
2.7. Quantitative Real-Time PCR
To confirm the mRNA expression levels of cisplatin-induced gene, total RNA was extracted from them using a Rneasy plus Mini Kit (Cat. No. 74134, Qiagen Inc., Germantown, MD, USA), cDNAs were synthesized from the total RNAs using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol. Real-time PCR was performed using an iQ SYBR-Green supermix Bio-Rad, Hercules, CA, USA). The fold change of gene expression was calculated using the 2−ΔΔCt method. We obtained the results by performing the experiment for 5 times.
Primers used for real-time PCR were as follows: CSF2 forward, 5′-atgcctgtcacgttgaatga-3′; reverse, 5′-ccgtagaccctgctcgaata-3′, FOS forward, 5′-gtccggttccttctatgcag-3′; reverse, 5′-taagtagtgcagcccggagt-3′, ATF3 forward, 5′-tgccaagtgtcgaaacaaga-3′; reverse, 5′-ccttcagctcagcattcaca-3′, JUN forward, 5′-agaggaagcgcatgaggaac-3′; reverse, 5′-ctgttccctgagcatgttgg-3′, ASK1 forward, 5′-gctcaagtcccagcccatag-3′; reverse, 5′-ctctcagccagccaggaagt -3′, TXNIP forward, 5′- taccccagaagctcctcctt-3′; reverse, 5′-gggctgtcttgagagtcgtc-3′, TXN1 forward, 5′-gcccttcttccattccctct-3′; reverse, 5′-aaggtcggcagcatttgact-3′, TXN2 forward, 5′-agaagatggtcgccaagcag-3′; reverse, 5′-ctggtcctcgtccttgatcc-3′, IL-6 forward, 5′-cacggccttccctacttcac-3′; reverse, 5′-ccacgatttcccagagaaca-3′, Nfkb forward, 5′-tctcaaagcagcaggagcag-3′; reverse, 5′-ggcaccactccctcatcttc-3′, TNF-α forward, 5′-agccgatgggttgtaccttg-3′; reverse, 5′-cggcagagaggaggttgact-3′, TP53 forward, 5′-acgggacagctttgaggttc-3′; reverse, 5′-gcagttcagggcaaaggact-3′, GAPDH forward, 5′-acccagaagactgtggatgg-3′; reverse, 5′-acacattgggggtaggaaca-3′. GAPDH was used as the reference gene.
2.8. Statistical Analysis
All values are represented as mean ± SD. For data analysis, we used the SPSS 23.0 statistical program. For the comparison of multiple groups in the caspase activities and qRT-PCR, ANOVA was used. A p value of <0.05 was considered statistically significant. For multiple comparisons, false discovery rate was used.
3. Results
3.1. Metformin Reduced Apoptosis and Increased Cell Survival
We analyzed the toxicity of cisplatin and the effect of metformin pretreatment in HEI-OC1 cell line. The cell viability was 53.15% in cisplatin group, but the cell viability increased to 69.30% when HEI-OC1 was pre-treated with metformin (Figure 1A), and the results were consistent with previous study [22]. In our previous study the pretreatment with metformin decreased apoptosis, reduced ROS production and lowered intracellular calcium concentration which were induced by cisplatin.
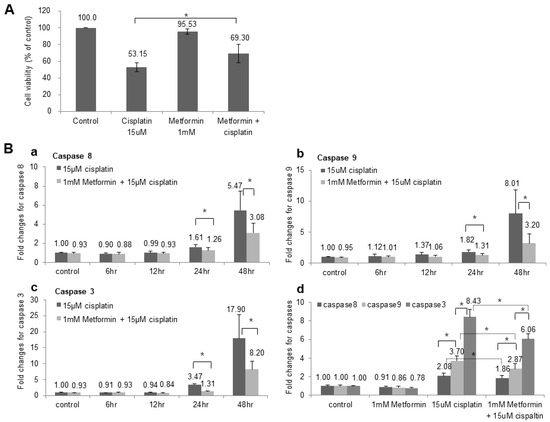
Figure 1.
Change of Cell viability and Caspase activity after metformin pretreatment. (A) The cell viability was 53.15% when HEI-OC1 cells were exposed to cisplatin for 48 h. However, the cell viability increased to 69.30% when HEI-OC1 was pretreated with metformin for 24 h prior to application of cisplatin. (B) (a) Caspase 8 activity started to increase at 24 h (1.61-fold change) after the cisplatin application and significantly increased after 48 h (5.47-fold change). Metformin pretreatment reduced the activity of caspase 8 in both 24 h (1.26-fold change) and 48 h (3.08-fold change) and these were statistically significant. * p < 0.05. (b) Caspase 9 activity was increased to 1.82-fold change at 24 h and 8.01-fold change at 48 h after the cisplatin application. Metformin pretreatment reduced the activity of caspase 9 in both 24 h (1.31-fold change) and 48 h (3.20-fold change) and these were statistically significant. (c) Caspase 3 activity was increased to 3.47-fold change at 24 h and 17.90-fold change at 48 h after the cisplatin application. Metformin pretreatment reduced the activity of caspase 3 in both 24 h (1.31-fold change) and 48 h (8.20-fold change) and these were statistically significant. (d) When we compared the activity level of caspase 8, 9 and 3, they were increased to 2.08-, 3.70- and 8.43-fold change, respectively, after application of cisplatin for 48 h. The activity level of all three caspases were decreased to 1.86-, 2.87-, and 6.06-fold change after the pretreatment with metformin for 24 h (n = 5).
We measured the caspase 8, 9 and 3 in time-dependent manner, and caspases started to increase at 24 h after the cisplatin application and significantly increased after 48 h. Metformin pretreatment reduced the activity of caspase 8, 9 and 3 in both 24 h and 48 h and these were statistically significant (Figure 1B). Additionally, when we compared the activity of caspase 8, 9 and 3, caspase 3 increased the most after cisplatin application followed by caspase 9 and 8 (Figure 1Bd). The fold changes of all three caspases were decreased after the pretreatment with metformin which indicates that metformin prevented cisplatin-induced apoptosis in both external and internal apoptotic pathway.
3.2. Cisplatin- and Metformin-Related Gene Expression Profile in HEI-OC1
To determine whether gene expression is altered in response to exposure to cisplatin and metformin pretreatment, we identified the gene expression after cisplatin exposure according to different timelines. A total of 89 genes were differentially expressed with |fold change| > 1.5 (p < 0.05) after 6 h, a total of 433 genes were differentially expressed after 15 h, a total of 941 genes were differentially expressed after 24 h, and a total of 2764 genes were differentially expressed after 48 h in cisplatin group (Figure 2A). Then, we identified the gene expression after cisplatin exposure in metformin pretreated groups according to different timelines. A total of 105 genes were differentially expressed with |fold change| > 1.5 (p < 0.05) after 6 h of cisplatin exposure, a total of 257 genes were differentially expressed after 15 h, a total of 1450 genes were differentially expressed after 24 h, and a total of 1463 genes were differentially expressed after 48 h (Figure 2A). Since the expression level of caspases were significant after 24 h and 48 h, differentially expressed genes (DEGs) of 24 h and 48 h were chosen for further analysis.
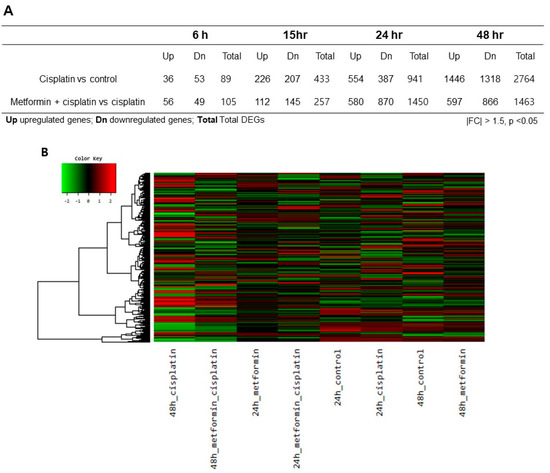
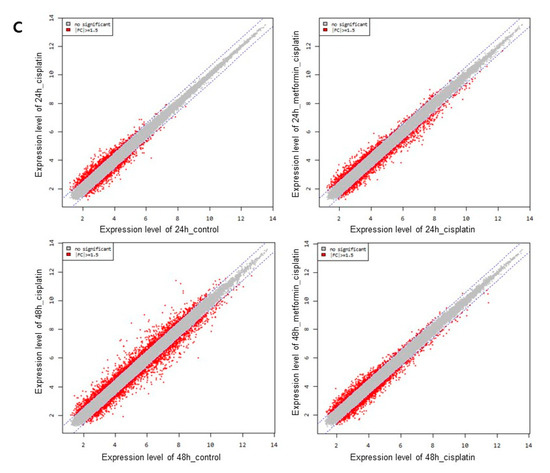
Figure 2.
Heatmap and volcano plots of RNA sequencing results. (A). A total of 89 genes were differentially expressed with |fold change| > 1.5 (p < 0.05) after 6 h, a total of 433 genes were differentially expressed after 15 h, a total of 941 genes were differentially expressed after 24 h, and a total of 2,764 genes were differentially expressed after 48 h in cisplatin group. When metformin was pretreated for 24 h prior to application of cisplatin, a total of 105 genes were differentially expressed with |fold change| > 1.5 (p < 0.05) after 6 h of cisplatin exposure, a total of 257 genes were differentially expressed after 15 h, a total of 1,450 genes were differentially expressed after 24 h, and a total of 1,463 genes were differentially expressed after 48 h. (B). In the heatmap, the DEGs between the various selected biological conditions were analyzed (|fold change| > 1.5 and p < 0.05). Red indicates transcripts with high expression, and green indicates transcripts with low expression. C. Plots of expression level were drawn for comparisons between the control and cisplatin group, the metformin pretreated group and cisplatin group.
To identify the differentially expressed genes (DEGs) between groups, we generated a heat map of the expression values of the selected DEGs comparing across genes and samples (|fold change| > 1.5 and p < 0.05). The DEGs between the various selected biological conditions (Figure 2B). Additionally, plots of expression level were drawn for comparisons between the control and cisplatin group; the metformin pretreated group and cisplatin group (Figure 2C). Cisplatin induced significant changes in gene expression after 24 h and 48 h exposure; metformin pretreatments also induced significant changes in gene expression.
KEGG pathway analysis revealed that cisplatin caused a change in the expression of genes involved in microRNAs in cancer, retinol metabolism, metabolic pathways in 24 h cisplatin group and metabolic pathways, microRNAs in cancer, PI3K-Akt signaling pathway, transcriptional misregulation in cancer, MAPK signaling pathway, pathways in cancer, axon guidance, proteoglycans in cancer, Rap1 signaling pathway, focal adhesion, p53 signaling pathway, cytokine–cytokine receptor interaction, TNF signaling pathway, endocytosis, FoxO signaling pathway and so on in 48 h cisplatin group (Tables S1 and S2). When metformin was pretreated, cisplatin caused a change in the expression of genes involved in transcriptional misregulation in cancer, microRNAs in cancer, PI3K-Akt signaling pathway, axon guidance, Rap1 signaling pathway, serotonergic synapse, focal adhesion, cytokine–cytokine receptor interaction, inflammatory mediator regulation of TRP channel, serotonergic synapse, metabolic pathways, p53 signaling pathway, pathways in cancer, tight junction and so on in 24 h metformin-pretreated group (Table 1, Table S3). Additionally, when metformin was pretreated, cisplatin caused a change in the expression of genes involved in microRNAs in cancer, cytokine–cytokine receptor interaction, phagosome, osteoclast differentiation, TNF signaling pathway in 48 h metformin-pretreated group (Table 2, Table S4).

Table 1.
Analysis of enrichment of KEGG pathways and involved genes in 24 h metformin-pretreated group.

Table 2.
Analysis of enrichment of KEGG pathways and involved genes in 48 h metformin-pretreated group.
For the 24 h metformin-pretreated group, the GO annotations of the predicted targets enriched among the 1450 genes that were mappable to DAVID, respectively, were selected according to a |fold-change| ≥ 1.5 and p-value ≤ 0.05 compared to the 24 h cisplatin group (Figure 3A). For the 48 h metformin-pretreated group, the GO annotations of the predicted targets enriched among the 1463 genes that were mappable to DAVID, respectively, were selected according to a |fold-change| ≥ 1.5 and p-value < 0.05 compared to the 48 h cisplatin group (Figure 3B). The functional annotation was categorized into biologic processes, cellular components and molecular functions, and only the top 10 GO terms showing the smallest p-values were considered.
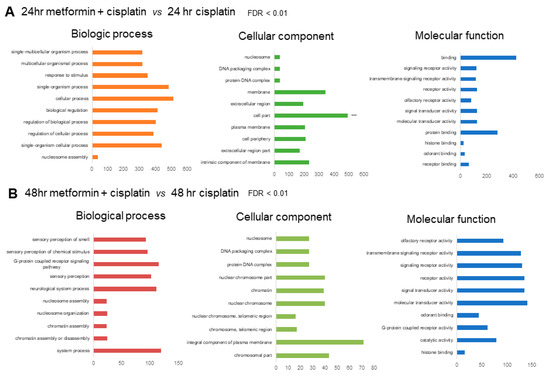
Figure 3.
(A). GO annotation of predicted targets in 24 h metformin-pretreated group compared to the 24 h cisplatin group. The top 10 most enriched GO terms are listed in terms for biological process, cellular component, and molecular function based on p-values. (B). GO annotation of predicted targets in 48 h metformin-pretreated group compared to the 48 h cisplatin group. The top 10 most enriched GO terms are listed in terms for biological process, cellular component, and molecular function based on FDR <0.01.
Among the genes affected in the 24 h cisplatin group, the top 20 upregulated genes included CYP2c38, Nlrp9c, and Cyp4a12 which are related to inflammation; Gzmf, and Gzme which are related to cell death; krt6b and Pcdhb8 which are related to cell adhesion. The downregulated genes included Gsdmc3 which is related to membrane permeabilization and pyroptosis; and Tlr8 which is related to innate and adaptive immunity (Table 3). Among the genes affected in the 24 h metformin-pretreated group, the top 20 upregulated genes included Cdkn1a, Btg2, Txnip, and Ccne1 which are related to cell cycle; Akr1c13 and Txnip which are related to oxidative stress; Trav6-3 which are related to immune response; S100a7a involved in inflammatory response, Trp53inp1involved in autophagic cell death. The top 20 downregulated genes included Cdh18 and Magi1 which are involved in cell–cell adhesion; Gpc6, Plxna2, and Ptprg which are related with cell migration; Camk1d involved in apoptosis; Dis3l2 and Cdk14 involved in cell cycle; Tbc1d5 involved in autophagy; and Prkca which is related with multiple biologic process such as cell adhesion, cell migration, apoptosis signaling, proliferation, inflammation (Table 4).

Table 3.
List of top 20 up and downregulated genes in 24 h cisplatin group.

Table 4.
List of top 20 up and downregulated genes in 24 h metformin-pretreated group.
Among the genes affected in the 48 h cisplatin group, the upregulated genes included Txnip, Btg2, and Cdkn1a which are related to cell cycle; Txnip which are related to oxidative stress; Trp53inp1 involved in autophagic cell death; Eda2r, Mdm2 and Fas which are involved in apoptotic cell death; Ptgs2 and S100a7a involved in inflammatory response; Fos involved in cell proliferation and differentiation; Tnfsf18 involved in T-cell responses; Egr1 which is related with the regulation of cell survival, proliferation and cell death. The top 20 downregulated genes included Ptn and Gas1 which are involved cell growth; Cdh18 and Magi1 which are involved in cell–cell adhesion (Table 5). Among the genes affected in the 48 h metformin-pretreated group, the top 20 upregulated genes included Cdsn involved in cell–cell adhesion; Ear1 and Mpeg1 involved in immune response; Eda related with cell death; Cd27 which is involved in apoptosis. The top 20 downregulated genes included Csf2, cytokine that stimulates the growth and differentiation of hematopoietic precursor cells from various lineages; Tnfsf18 which is involved in T-cell response; Fos and Egr1 which are related with cell proliferation and death, Btg2 involved with cell cycle regulation; Fos and Nr4a2 which are involved in cellular response to oxidative stress (Table 6).

Table 5.
List of top 20 up and downregulated genes in 48 h cisplatin group.

Table 6.
List of top 20 up and downregulated genes in 48 h metformin-pretreated group.
To identify whether metformin pretreatment caused the gene expression opposite to the cisplatin alone treated group, we’ve selected top 30 upregulated and downregulated genes in both 24 h and 48 h metformin pretreatment groups and evaluated the gene expression level of the 24 h and 48 h cisplatin groups and drawn heat maps (Figure 4). Pretreatment of metformin for 24 h increased the expression of Tagap1, Akr1c13, Trav6-3, Fbxw20, Scn10a, Uckl1os, Tlr8, and Mterf1a more than |fold-change| ≥ 1.5 while significantly decreasing same genes with |fold-change| ≥ 1.5 in cisplatin alone group (Figure 4A). However, top 30 downregulated genes in 24 h metformin pretreatment group were not in accord with elevated genes in cisplatin alone group (Figure 4B). Pretreatment of metformin for 48 h increased the expression of Taar7b, H2-DMb2, Rhox3f, Ear1, Krt6b, Snord98, Eda, Atp6v1g3, Mpeg1, Traj25, Ffar2, Dlx6os1, Zfp438, Gzmf, Aldh1l2, Magi2, Ifna11, Tfaj43, and Snord52 more than |fold-change| ≥ 1.5 while significantly decreasing same genes with |fold-change| ≥ 1.5 in 48 h cisplatin group (Figure 4C). Likewise, pretreatment of metformin for 48 h decreased the expression of Csf2, Slc40a1, Snora75, Tnfsf18, Tcrg-C4, Fos, Btg2, Egr1, Gpx2-ps1, Rhox4f, Ampd1, Nr4a2, Txnip, Gabra4 and Klk1b26 more than |fold-change| ≥ 1.5 while significantly increasing same genes with |fold-change| ≥ 1.5 in the 48 h cisplatin group (Figure 4D).
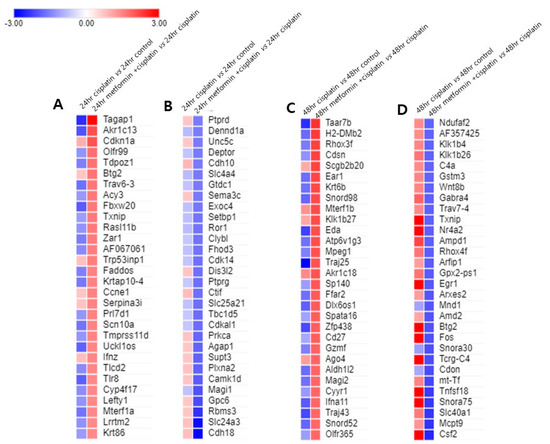
Figure 4.
Metformin pretreatment-triggered gene expression changes compared to cisplatin group. (A). The expression level of top 30 upregulated genes in 24 h metformin-pretreated group were compared with those of 24 h cisplatin group. Pretreatment of metformin for 24 h increased the expression of Tagap1, Akr1c13, Trav6-3, Fbxw20, Scn10a, Uckl1os, Tlr8, and Mterf1a more than |fold-change| ≥ 1.5 while significantly decreasing same genes with |fold-change| ≥ 1.5 in cisplatin alone group. (B). The expression level of top 30 downregulated genes in 24 h metformin-pretreated group were not in accord with elevated genes in 24 h cisplatin group. (C). Pretreatment of metformin for 24 h and then exposure to cisplatin for 48 h (48 h metformin-pretreated group) increased the expression of Taar7b, H2-DMb2, Rhox3f, Ear1, Krt6b, Snord98, Eda, Atp6v1g3, Mpeg1, Traj25, Ffar2, Dlx6os1, Zfp438, Gzmf, Aldh1l2, Magi2, Ifna11, Tfaj43, and Snord52 more than |fold-change| ≥ 1.5 while significantly decreasing same genes with |fold-change| ≥ 1.5 in 48 h cisplatin group. (D). Likewise, in 48 h metformin-pretreated group, the expression of Csf2, Slc40a1, Snora75, Tnfsf18, Tcrg-C4, Fos, Btg2, Egr1, Gpx2-ps1, Rhox4f, Ampd1, Nr4a2, Txnip, Gabra4 and Klk1b26 decreased more than |fold-change| ≥ 1.5 while significantly increasing same genes with |fold-change| ≥ 1.5 in the 48 h cisplatin group. Red indicates transcripts with high expression, and blue indicates transcripts with low expression.
3.3. Network Analysis and qRT-PCR Expression Levels of the Potential Biomarkers
The molecular signaling networks among the genes that were differentially expressed in response to application of cisplatin and metformin were analyzed to predict the relevant molecular pathways. The analysis was performed among genes that were significantly expressed oppositely in response to cisplatin and the application of metformin (Table S5, Table 7). Various genes such as Fos, Fosl1, Csf2, Cxcl1, Egr1, Btg2, Atf3, and Nr4a2 were identified to be related to each other in 48 h groups (Figure 5A).

Table 7.
List of involved genes which are expressed oppositely in 48 h cisplatin group and 48 h metformin pretreated group.
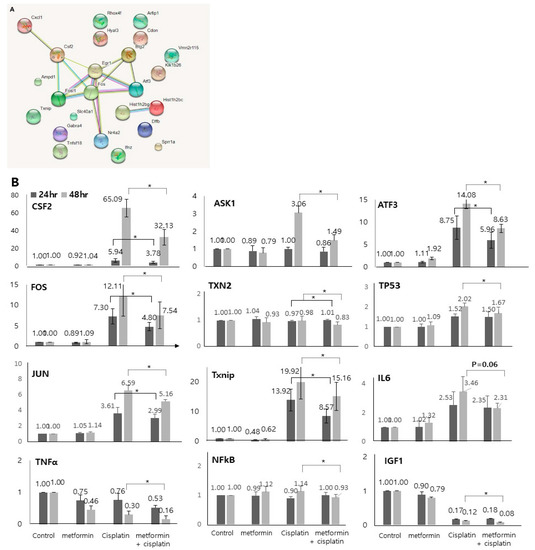
Figure 5.
Network analysis and qRT-PCR expression levels of the potential biomarkers. (A). The network analysis was performed among genes that were significantly expressed oppositely in response to cisplatin and the application of metformin (48 cisplatin group vs. 48 h metformin-pretreated group). Various genes such as Fos, Fosl1, Csf2, Cxcl1, Egr1, Btg2, Atf3, and Nr4a2 were identified to be related to each other. (B) 48 h cisplatin exposure-induced and metformin-pretreated prior to 48 h cisplatin exposure-induced genes validated by qRT-PCR. In accordance with the microarray results, the expression levels of CSF2, FOS, JUN, TNFα, NFκB, Txnip, ASK1, TXN2, ATF3, TP53, and IGF were found to be decreased after application of metformin (p < 0.05). The expression levels of IL6 decreased after application of metformin but it was not statistically significant (p = 0.06). Solid lines show the statistics between 24 h cisplatin group and 24 metformin-pretreated group. Dotted lines show the statistics between 48 h cisplatin group and 24 metformin-pretreated group (n = 5).
Based on the gene expression and network analyses, we investigated several genes (CSF2, FOS, JUN, TNFα, NFκB, Txnip, ASK1, TXN2, ATF3, TP53, IL6, and IGF1) as metformin-related preventive biomarkers in cisplatin ototoxicity. The selection of key gens was performed based on the analysis of genes that were oppositely expressed in response to cisplatin and the application of metformin (Table 7), and on the analysis of genes that were most up and downregulated after 48 h treatment with cisplatin with or without application of metformin (Table 5 and Table 6) and on well-known related genes. To validate the microarray results, we examined the expressed transcript levels by qRT-PCR. In accordance with the RNA sequencing results, the expression levels of CSF2 (5.94 vs. 3.78, p = 0.006 for 24 h; 65.09 vs. 32.13, p = 0.003 for 48 h), FOS (7.30 vs. 4.80, p = 0.005 for 24 h; 12.00 vs. 7.54, p = 0.000 for 48 h), JUN (3.61 vs. 2.99, p = 0.043 for 24 h; 6.59 vs. 5.16, p = 0.007 for 48 h), TNFα (0.30 vs. 0.16, p = 0.008 for 48 h), NFκB (1.14 vs. 0.93, p = 0.031 for 48 h), Txnip (13.92 vs. 8.57, p = 0.005 for 24 h; 19.92 vs. 15.16, p = 0.002 for 48 h), ASK1 (3.06 vs. 1.49, p = 0.000 for 48 h), TXN2 (0.97 vs. 1.01, p = 0.026 for 24 h; 0.98 vs. 0.83, p = 0.047 for 48 h), ATF3 (8.75 vs. 5.96, p = 0.006 for 24 h; 14.08 vs. 8.63, p = 0.000 for 48 h), TP53 (2.02 vs. 1.67, p = 0.021 for 48 h), and IGF (1.12 vs. 0.08, p = 0.035 for 48 h) were found to be decreased after application of metformin (Figure 5B). The expression levels of IL6 decreased after application of metformin but it was not statistically significant (3.46 vs. 2.31, p = 0.060 for 48 h).
4. Discussion
In this study, we evaluated the change of gene expression induced by cisplatin at several different time points. We identified that cisplatin application caused delayed gene expression changes, which means that as the exposure time to cisplatin lengthened, the number of affected genes increased. Genes which were upregulated or downregulated after 24 h exposure to cisplatin were genes that were related with inflammation (CYP2c38, Nlrp9c, and Cyp4a12), cell adhesion (krt6b and Pcdhb8), membrane permeabilization and pyroptosis (Gsdmc3), and cell death (Gzmf and Gzme). Exposure to cisplatin for 48 h altered genes that were related with cell cycle (Txnip, Btg2, and Cdkn1a), oxidative stress (Txnip), inflammatory process (Ptgs2 and S100a7a), cell proliferation and cell death (Fos, Egr1), autophagic cell death (Trp53inp1), and apoptotic cell death (Eda2, Mdm2 and Fas). These data support the cellular change at the gene level after the application of cisplatin.
There are several reports demonstrating the gene expression change in cisplatin-induced ototoxicity in HEI-OC1 cells using RNA sequencing analysis [24,25]. In a study evaluating the gene expression change after 30 h cisplatin exposure, downregulated DEGs were associated with several KEGG pathways such as ‘systemic lupus erythematosus’, ‘alcoholism’, ‘viral carcinogenesis’, ‘PI3K-Akt signaling pathway’, ‘Rap1 signaling pathway’, and ‘HIF-1 signaling pathway’; which are in accord with our data (Supplementary Table S2). They also reported that upregulated DEGs were significantly associated with autophagy, apoptosis-associated processes, response to DNA damage and cell cycle arrest; and demonstrated several genes such as phorbol-12-myristate-13-acetate-induced protein 1 (PMAIPI), Bcl-2 binding component 3 (BBC3), zinc finger matrin-type 3 (ZMAT3), p53-induced death domain protein 1 (PIDD1), B-cell translocation gene protein 2 (BTG2), thioredoxin-inter-acting protein (TXNIP), DNA damage induced apoptosis suppressor (DDIAS), cycle-dependent kinase inhibitor 1a (Cdkn1a), transformation related protein 53-induced nuclear protein 1 (Trp53inp1), Foxo3, and Fas; which are also similar to our results.
In our previous study, pretreatment with metformin prior to the application of cisplatin significantly decreased the late apoptosis in HEI-OCI cells [22]. Metformin attenuated the cisplatin-induced increase in ROS, inhibited the activation of caspase-3 and levels of poly-ADP-ribose polymerase (PARP) and prevented the cisplatin-induced elevation in intracellular calcium concentrations after 48 h exposure to cisplatin. In this study, we measured the caspase 8, 9 and 3 in time-dependent manner, and identified that caspases started to increase at 24 h after the cisplatin application and significantly increased after 48 h. Metformin pretreatment reduced the expression of caspase 8, 9 and 3 in both 24 h and 48 h, indicating that metformin is involved in both intrinsic and extrinsic apoptotic pathways [26].
We identified several genes (CSF2, FOS, JUN, TNFα, NFκB, Txnip, ASK1, TXN2, ATF3, TP53, IL6, and IGF1) which are related with the preventive effects of metformin in cisplatin ototoxicity based on the gene expression and network analyses. Among these genes, thioredoxin-interacting protein (Txnip) is reported to have relation with cell apoptosis and inflammation [27,28]. The primary role of Txnip is inhibition of thioredoxin (TRX), an important redox protein which controls levels of reactive oxygen species, and the inhibition of TRX by Txnip promotes inflammation and increases levels of ROS [29,30]. Additionally, the redox-regulated apoptosis-signal kinase (ASK1), a member of the mitogen- activated protein kinase family, is considered as an important link between cellular stress and innate immunity [31,32]. ASK1 is usually bound to mitochondrial thioredoxin (TRX2) under normal conditions, but, during stress and following Txnip translocation to the mitochondria, ASK1-TRX2 binding is interrupted and triggers an apoptotic signal cascade leading to cytochrome c release and caspase-3 cleavage, and apoptosis [33].
Metformin is known to exert direct immunomodulatory effect on immune cells by AMPK induction and mTORC1 inhibition. The mTOR is subcategorized into two multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which could be differentiated based on their related protein [34]. mTORC1 is responsible for cell growth and proliferation in response to growth factors and nutrient while mTORC2 is insensitive to nutrients. The two main downstream targets of mTORC1 are p70S6 kinase (S6K) and elongation factor 4E binding protein (4E-BP1) [35]; although mTORC2 is relatively unknown, they are reported to be related with PI3K, and Akt [36].
There are relatively many studies regarding the role of mTOR in cancer, but it is not much known about the role of mTOR in inner ear. Recent study reported that a low dose intraperitoneal injection of sirolimus (mTOR inhibitor) attenuated age-related hearing loss by decreasing the mTORC1, while high dose of sirolimus caused severe hearing loss by decreasing mTORC2/Akt. They also reported that mTORC2/Akt is involved in the regulation of hair cell survival in the cisplatin exposure condition, suggesting that the therapeutic activation of mTORC2 in conjunction with decreasing mTORC1 might represent a promising and effective strategy in preventing hearing loss [37].
In pancreatic β cell, mTOR is reported to associate with the carbohydrate-response element–binding protein (ChREBP)–Max-like protein complex and inhibit its transcriptional activity, leading to decreased expression of TXNIP, a potent inducer of pancreatic β cell death and oxidative stress. Meanwhile, mTOR inhibitor or mTOR deficiency enhances transcriptional activity and activates Txnip expression [38]. Additionally, mTOR and HDAC inhibitors converge on the TXNIP/thioredoxin pathway and cause oxidative stress and apoptosis in esophageal cancer [39].
However, in our study, we identified that metformin, which is mTOR inhibitor, decreased the expression level of Txnip compared to the level evoked by cisplatin. Cisplatin increased the expression of Txnip in 24 h and 48 h exposure group, but pretreatment with metformin reduced the Txnip expression in HEI-OC1 cells. ASK1 was also elevated after 48 h application of cisplatin but when HEI-OC1 cells were pretreated with metformin prior to application of cisplatin, the level of both Txnip and ASK1 reduced. Previous studies also demonstrated that metformin significantly reduced Txnip mRNA and protein expression [40]. Although, our experiment was performed on HEI-OC1 cell line and further validation studies are required in the future, our observation suggests that metformin prevented the inhibitory effect of Txnip which primary role is to inhibit TRX, lowered levels of ROS, and reduced apoptotic cascade signals by decreasing ASK1 expression which would lead to caspase-3 cleavage and apoptosis.
5. Conclusions
In this study, we evaluated the change of gene expression induced by cisplatin at several different time points, and the alteration of gene expression according to pretreatment with metformin in HEI-OC1 cells through microarray analysis. We have identified potential molecular modulator genes (CSF2, FOS, JUN, TNFα, NFκB, Txnip, ASK1, TXN2, ATF3, TP53, IL6, and IGF1) that might be related with the preventive role of metformin in the cisplatin ototoxicity. Additionally, our observation suggests that metformin would prevent the expression of Txnip and ASK1 and thus lowers ROS levels, and reduces apoptotic cascade signals in cisplatin-induced ototoxicity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb45010021/s1. Supplementary Table S1. Analysis of enrichment of KEGG pathways and involved genes in 24 h cisplatin group. Supplementary Table S2. Analysis of enrichment of KEGG pathways and involved genes in 48 h cisplatin group. Supplementary Table S3. Involved genes which are expressed oppositely in 24 h cisplatin group and 24 h metformin pretreated group.
Author Contributions
Conceptualization, S.L. and J.C.; methodology, S.L. and J.C.; data analysis, S.C. and J.C.; sample acquisition, S.L.; data curation, S.C. and S.H.P.; writing—original draft preparation, S.L. and J.C.; writing—review and editing, G.J.I. and J.C.; supervision, G.J.I. and J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF-2021R1A2C2008293) and Bumsuk Academic Research Fund (5S140101542S000100).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mckeage, M.J. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995, 13, 228–244. [Google Scholar] [CrossRef]
- Marshak, T.; Steiner, M.; Kaminer, M.; Levy, L.; Shupak, A. Prevention of Cisplatin-Induced Hearing Loss by Intratympanic Dexamethasone: A Randomized Controlled Study. Otolaryngol. Head Neck Surg. 2014, 150, 983–990. [Google Scholar] [CrossRef]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front. Cell. Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Hazlitt, R.A.; Min, J.; Zuo, J. Progress in the development of preventative drugs for cisplatin-induced hearing loss. J. Med. Chem. 2018, 61, 5512–5524. [Google Scholar] [CrossRef] [PubMed]
- So, H.S.; Kim, H.J.; Lee, J.H.; Park, S.Y.; Park, C.; Kim, Y.H.; Kim, J.K.; Lee, K.M.; Kim, K.S.; Chung, S.Y.; et al. Flunarizine induces Nrf2-mediated transcriptional activation of heme oxygenase-1 in protection of auditory cells from cisplatin. Cell Death Differ. 2006, 13, 1763–1775. [Google Scholar] [CrossRef]
- So, H.S.; Kim, H.J.; Kim, Y.; Kim, E.; Pae, H.O.; Chung, H.T.; Kim, H.J.; Kwon, K.B.; Lee, K.M.; Lee, H.Y.; et al. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J. Assoc. Res. Otolaryngol. 2008, 9, 290–306. [Google Scholar] [CrossRef]
- Kaur, T.; Borse, V.; Sheth, S.; Sheehan, K.; Ghosh, S.; Tupal, S.; Jajoo, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J. Neurosci. 2016, 36, 3962–3977. [Google Scholar] [CrossRef]
- Ghosh, S.; Sheth, S.; Sheehan, K.; Mukherjea, D.; Dhukhwa, A.; Borse, V.; Rybak, L.P.; Ramkumar, V. The Endocannabinoid/Cannabinoid Receptor 2 System Protects Against Cisplatin-Induced Hearing Loss. Front. Cell. Neurosci. 2018, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Chen, L.; Krailo, M.D.; Knight, K.; Villaluna, D.; Bliss, B.; Pollock, B.H.; Ramdas, J.; Lange, B.; Van Hoff, D.; et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 63–74. [Google Scholar] [CrossRef]
- Bouffet, E. Reducing cisplatin ototoxicity in children: Some hope and many questions. Lancet Oncol. 2017, 18, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Sarafraz, Z.; Ahmadi, A.; Daneshi, A. Transtympanic injections of N-acetylcysteine and dexamethasone for prevention of cisplatin-induced ototoxicity: Double blind randomized clinical trial. Int. Tinnitus J. 2018, 22, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.G.; Chelis, L.; Kakolyris, S.; Papadopoulos, S.; Stathakidou, S.; Chamalidou, E.; Xenidis, N.; Amarantidis, K.; Dimopoulos, P.; Danielides, V. Transtympanic injections of N-acetylcysteine for the prevention of cisplatin-induced ototoxicity: A feasible method with promising efficacy. Am. J. Clin. Oncol. 2013, 6, 1–6. [Google Scholar] [CrossRef]
- Yoo, J.; Hamilton, S.J.; Angel, D.; Fung, K.; Franklin, J.; Parnes, L.S.; Lewis, D.; Venkatesan, V.; Winquist, E. Cisplatin otoprotection using transtympanic L-N-acetylcysteine: A pilot randomized study in head and neck cancer patients. Laryngoscope 2014, 124, E87–E94. [Google Scholar] [CrossRef]
- Foster-Nora, J.A.; Siden, R. Amifostine for protection from antineoplastic drug toxicity. Am. J. Health Syst. Pharm. 1997, 54, 787–800. [Google Scholar] [CrossRef]
- Fouladi, M.; Chintagumpala, M.; Ashley, D.; Kellie, S.; Gururangan, S.; Hassall, T.; Gronewold, L.; Stewart, C.F.; Wallace, D.; Broniscer, A.; et al. Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma. J. Clin. Oncol. 2008, 26, 3749–3755. [Google Scholar] [CrossRef]
- Gurney, J.G.; Bass, J.; Onar-Thomas, A.; Huang, J.; Chintagumpala, M.; Bouffet, E.; Hassall, T.; Gururangan, S.; Heath, J.A.; Kellie, S.; et al. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro-Oncology 2014, 16, 848–855. [Google Scholar] [CrossRef]
- Villani, V.; Zucchella, C.; Cristalli, G.; Galiè, E.; Bianco, F.; Giannarelli, D.; Carpano, S.; Spriano, G.; Pace, A. Vitamin E neuroprotection against cisplatin ototoxicity: Preliminary results from a randomized, placebo-controlled trial. Head Neck 2016, 38, E2118–E2121. [Google Scholar] [CrossRef] [PubMed]
- El-Mir, M.Y.; Detaille, D.; R-Villanueva, G.; Delgado-Esteban, M.; Guigas, B.; Attia, S.; Fontaine, E.; Almeida, A.; Leverve, X. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J. Mol. Neurosci. 2008, 34, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Le Marchand-Brustel, Y.; Tanti, J.F.; Bost, F. Metformin in cancer therapy: A new perspective for an old antidiabetic drug? Mol. Cancer Ther. 2010, 9, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021, 12, 718942. [Google Scholar] [CrossRef]
- Sorrenti, V.; Benedetti, F.; Buriani, A.; Fortinguerra, S.; Caudullo, G.; Davinelli, S.; Zella, D.; Scapagnini, G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals 2022, 15, 912. [Google Scholar] [CrossRef]
- Chang, J.; Jung, H.H.; Yang, J.Y.; Lee, S.; Choi, J.; Im, G.J.; Chae, S.W. Protective effect of metformin against cisplatin-induced ototoxicity in an auditory cell line. J. Assoc. Res. Otolaryngol. 2014, 15, 149–158. [Google Scholar] [CrossRef][Green Version]
- Kalinec, G.M.; Webster, P.; Lim, D.J.; Kalinec, F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol. Neurootol. 2003, 8, 177–189. [Google Scholar] [CrossRef]
- Gaun, G.; He, X.; Chen, J.; Bin, L.; Tang, X. Identifying the mechanisms underlying the protective effect of tetramethylpyrazine against cisplatin-induced in vitro ototoxicity in HEI-OC1 auditory cells using gene expression profiling. Mol. Med. Rep. 2020, 22, 5033–5068. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, Z.; Li, W.; Tang, D.; Zhao, L.; He, Y.; Li, H. Inhibition of KDM5A attenuates cisplatin-induced hearing loss via regulation of the MAPK/AKT pathway. Cell. Mol. Life Sci. 2022, 79, 596. [Google Scholar] [CrossRef]
- Denicourt, C.; Dowdy, S.F. Medicine. Targeting apoptotic pathways in cancer cells. Science 2004, 305, 1411–1413. [Google Scholar] [CrossRef]
- Anu Shah, A.; Xia, L.; Goldberg, H.; Lee, K.W.; Quaggin, S.E.; Fantus, I.G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013, 288, 6835–6848. [Google Scholar] [CrossRef]
- Berk, B.D. Novel approaches to treat oxidative stress and cardiovascular diseases. Trans. Am. Clin. Climatol. Assoc. 2007, 118, 209–214. [Google Scholar]
- Junn, E.; Han, S.H.; Im, J.Y.; Yang, Y.; Cho, E.W.; Um, H.D.; Kim, D.K.; Lee, K.W.; Han, P.L.; Rhee, S.G.; et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J. Immunol. 2000, 164, 6287–6295. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rong, Y.; Zhang, M.; Wang, X.L.; LeMaire, S.A.; Coselli, J.S.; Zhang, Y.; Shen, Y.H. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem. Biophys. Res. Commun. 2009, 381, 660–665. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef]
- Kolliputi, N.; Waxman, A.B. IL-6 cytoprotection in hyperoxic acute lung injury occurs via suppressor of cytokine signaling-1-induced apoptosis signal-regulating kinase-1 degradation. Am. J. Respir. Cell Mol. Biol. 2009, 40, 314–324. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pathak, S.; Basak, C.; Law, S.; Kundu, M.; Basu, J. Execution of macrophage apoptosis by Mycobacterium avium through apoptosis signal-regulating kinase 1/p38 mitogen-activated protein kinase signaling and caspase 8 activation. J. Biol. Chem. 2003, 278, 26517–26525. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.; Guertin, D.A.; Latek, R.R.; Erdjument-Gromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Zinzalla, V.; Stracka, D.; Oppliger, W.; Hall, M.N. Activation of mTORC2 by association with the ribosome. Cell 2011, 144, 757–768. [Google Scholar] [CrossRef]
- Fu, X.; Li, P.; Zhang, L.; Song, Y.; An, Y.; Zhang, A.; Liu, W.; Ye, C.; Zhang, Y.; Yue, R.; et al. Activation of Rictor/mTORC2 signaling acts as a pivotal strategy to protect against sensorineural hearing loss. Proc. Natl. Acad. Sci. USA 2022, 119, e2107357119. [Google Scholar] [CrossRef]
- Chau, G.C.; Im, D.U.; Kang, T.M.; Bae, J.M.; Kim, W.; Pyo, S.; Moon, E.Y.; Um, S.H. mTOR controls ChREBP transcriptional activity and pancreatic β cell survival under diabetic stress. J. Cell Biol. 2017, 216, 2091–2105. [Google Scholar] [CrossRef]
- Malone, C.F.; Emerson, C.; Ingraham, R.; Barbosa, W.; Guerra, S.; Yoon, H.; Liu, L.L.; Michor, F.; Haigis, M.; Macleod, K.F.; et al. mTOR and HDAC inhibitors converge on the TXNIP/thioredoxin pathway to cause catastrophic oxidative stress and regression of RAS-driven tumors. Cancer Discov. 2017, 7, 1450–1463. [Google Scholar] [CrossRef]
- Chai, T.F.; Hong, S.Y.; He, H.; Zheng, L.; Hagen, T.; Luo, Y.; Yu, F.-X. A potential mechanism of metformin-mediated regulation of glucose homeostasis: Inhibition of Thioredoxin-interacting protein (Txnip) gene expression. Cell. Signal. 2012, 24, 1700–1705. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).