Molecular Characterization of Two Genes Encoding Novel Ca2+-Independent Phospholipase A2s from the Silkworm, Bombyx mori
Abstract
:1. Introduction
2. Materials and Methods
2.1. Exprimental Animals
2.2. RNA Extraction and cDNA Synthesis
2.3. Gene Amplification
2.4. Bioinformatic Analysis
2.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Culture of Microbial Pathogens and Pathogen Stimulation
2.7. 20-Hydroxyecdysone Induction
2.8. Statistical Analysis
3. Results
3.1. Subsection
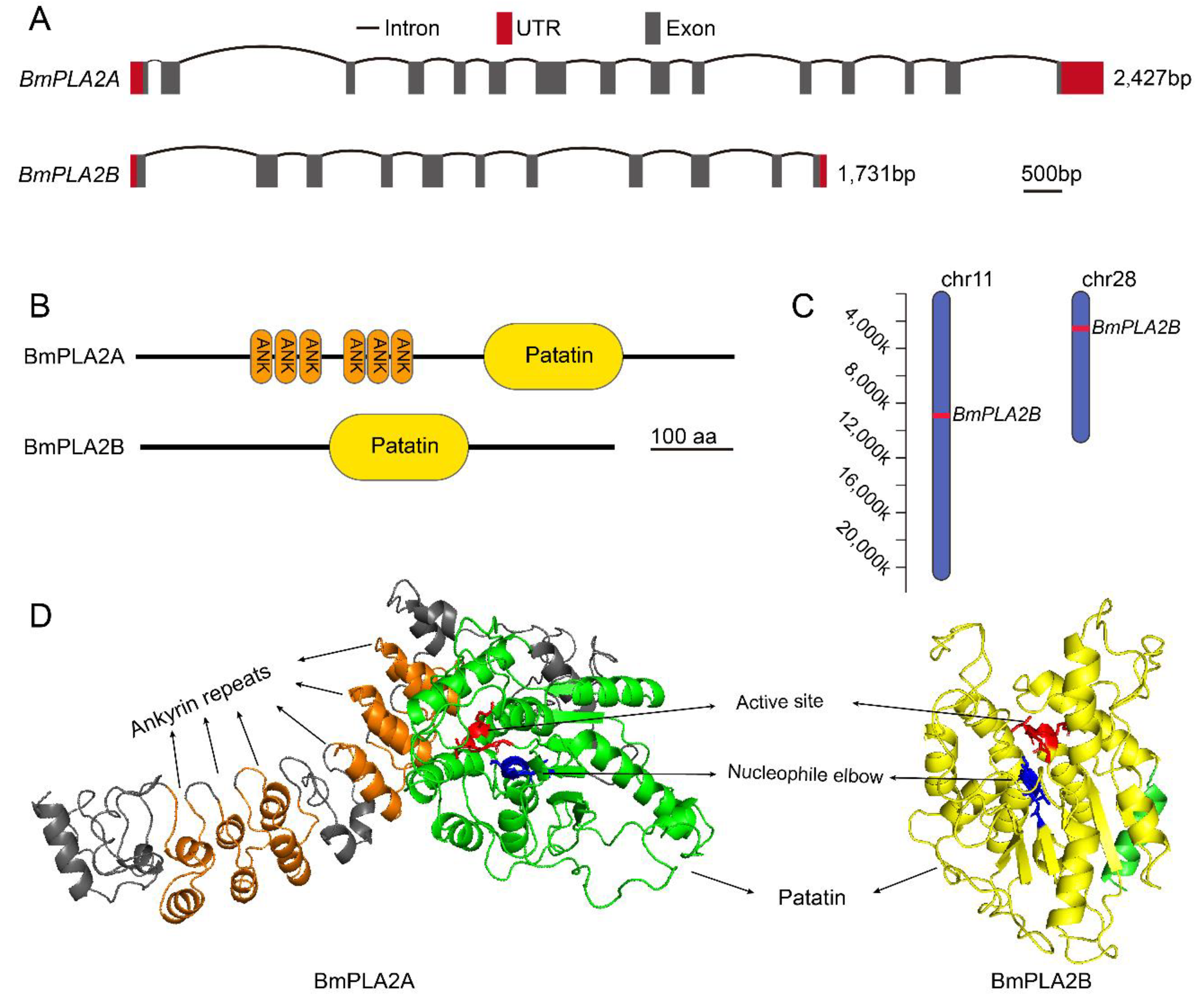
3.1.1. Molecular Cloning and Amino Acid Sequence Analysis of BmiPLA2 Genes in Silkworm
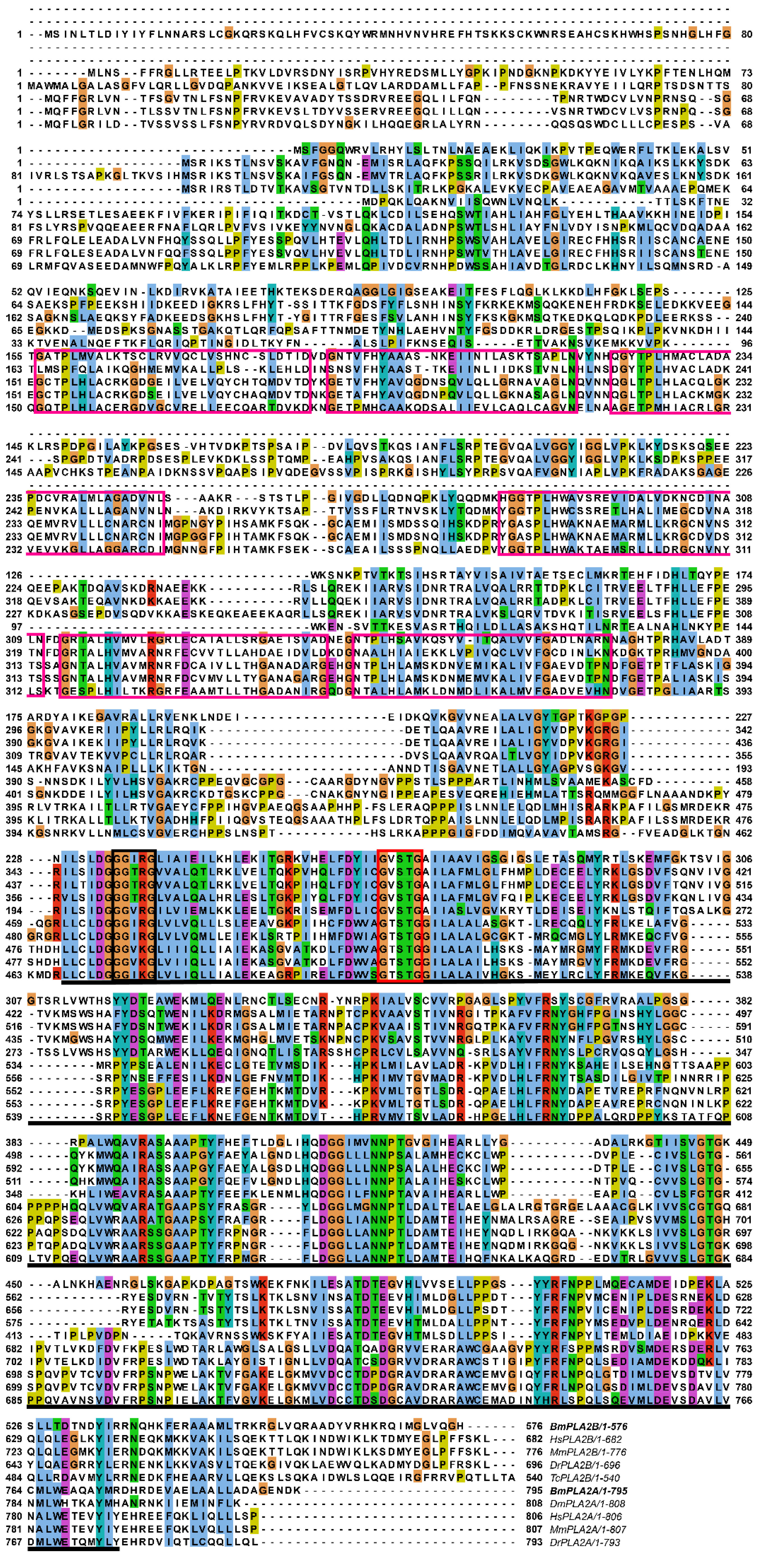
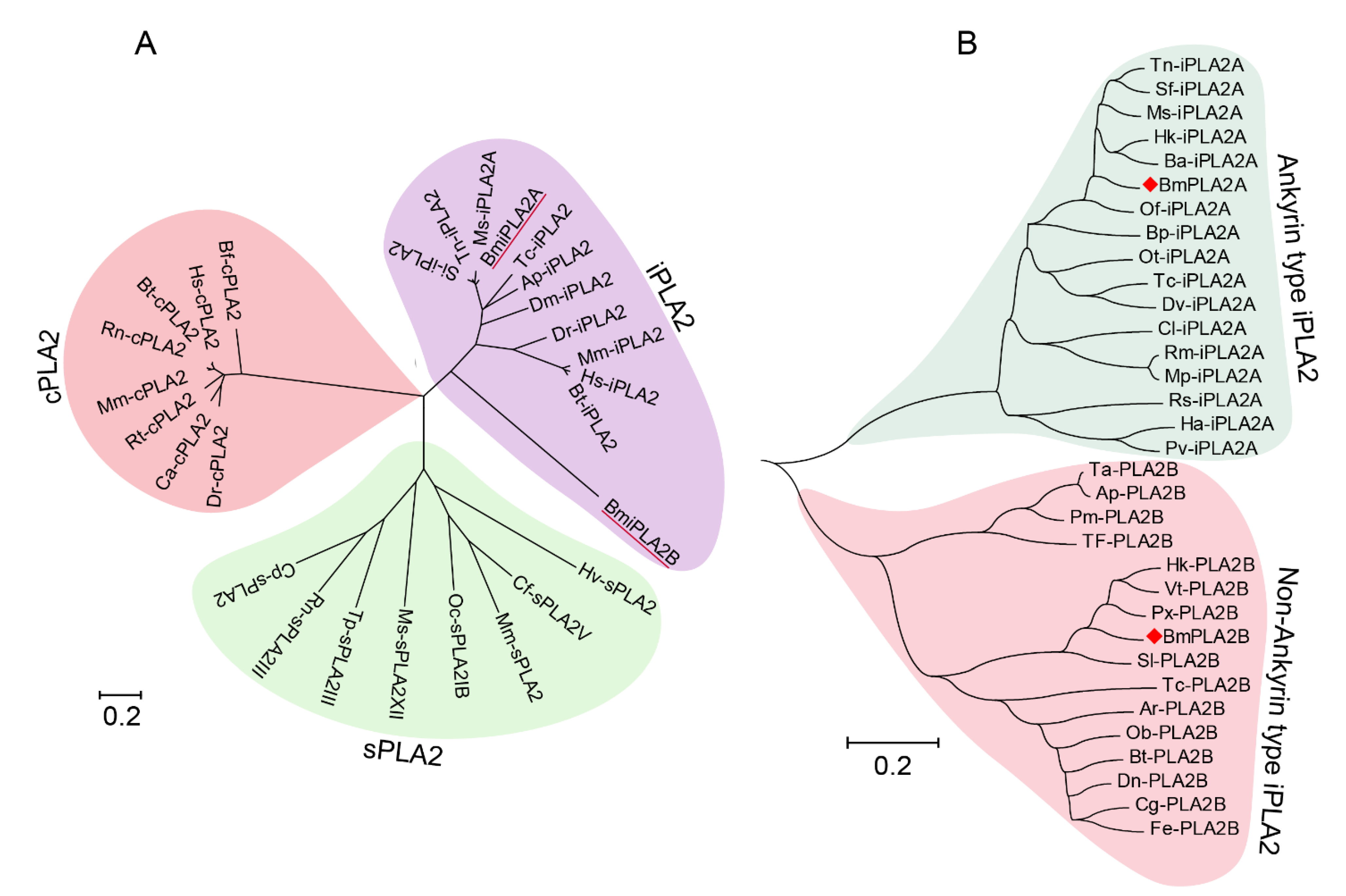
3.1.2. Conserved Analysis and Phylogenetic Analysis of BmiPLA2s Protein in Silkworm
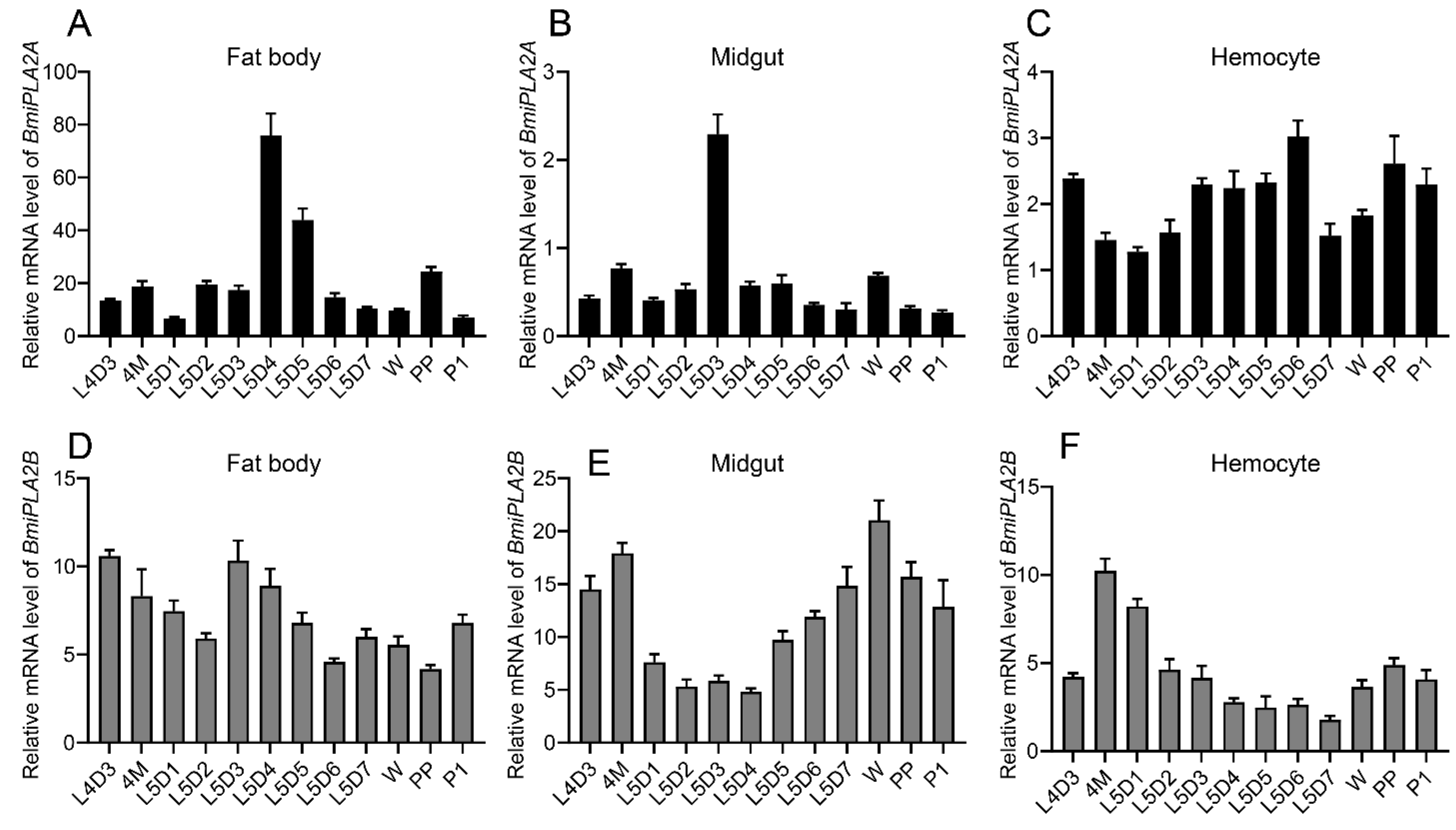
3.1.3. Tissue Distribution of BmiPLA2s
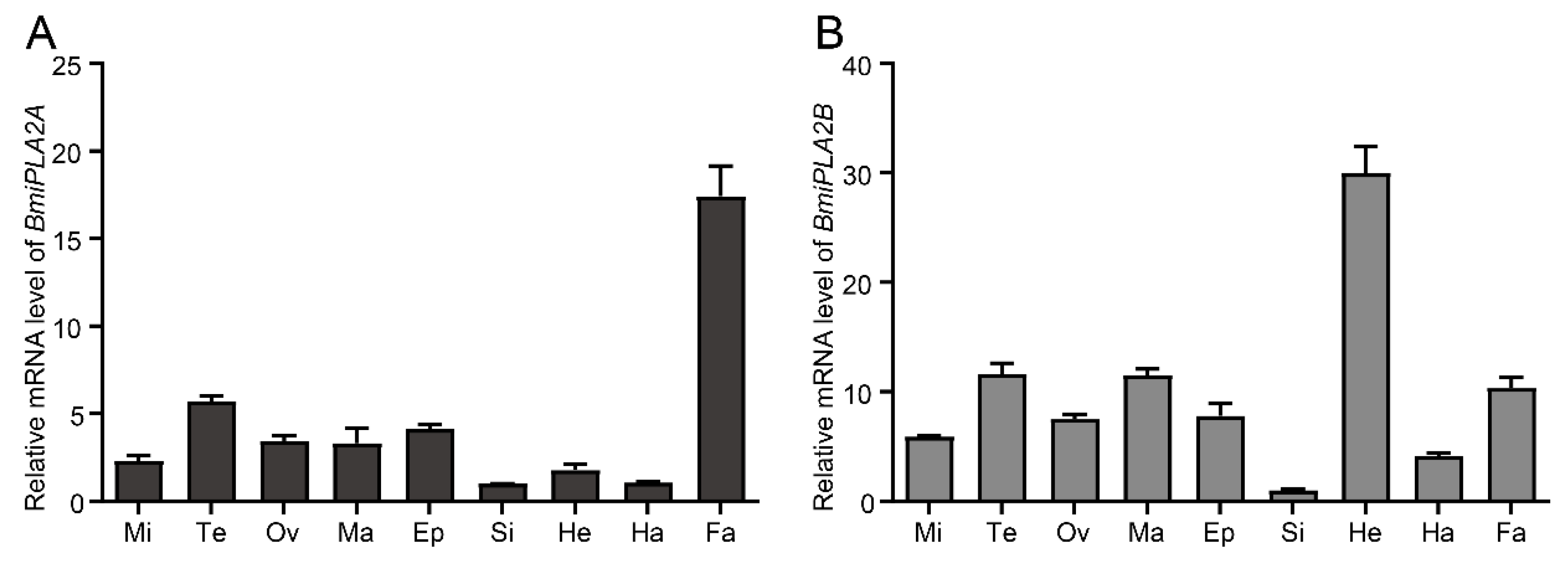
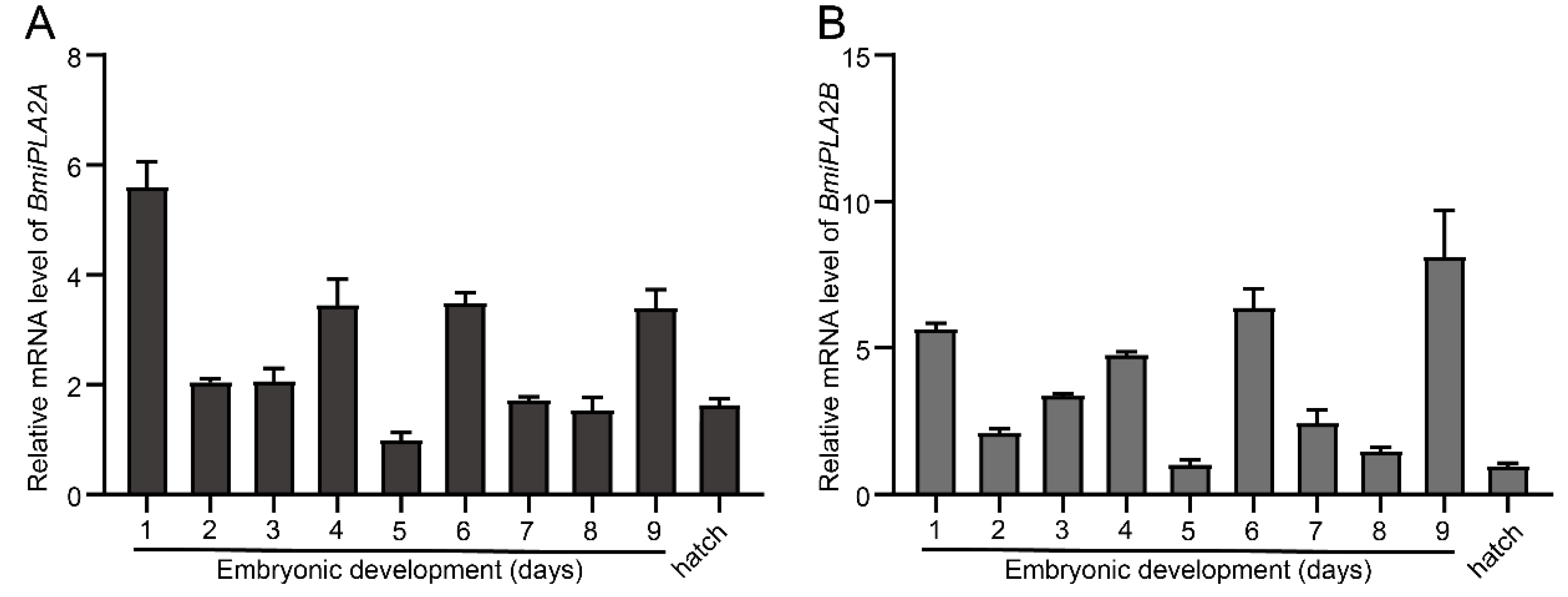
3.1.4. Temporal Expression Profiles of BmiPLA2s in Silkworm
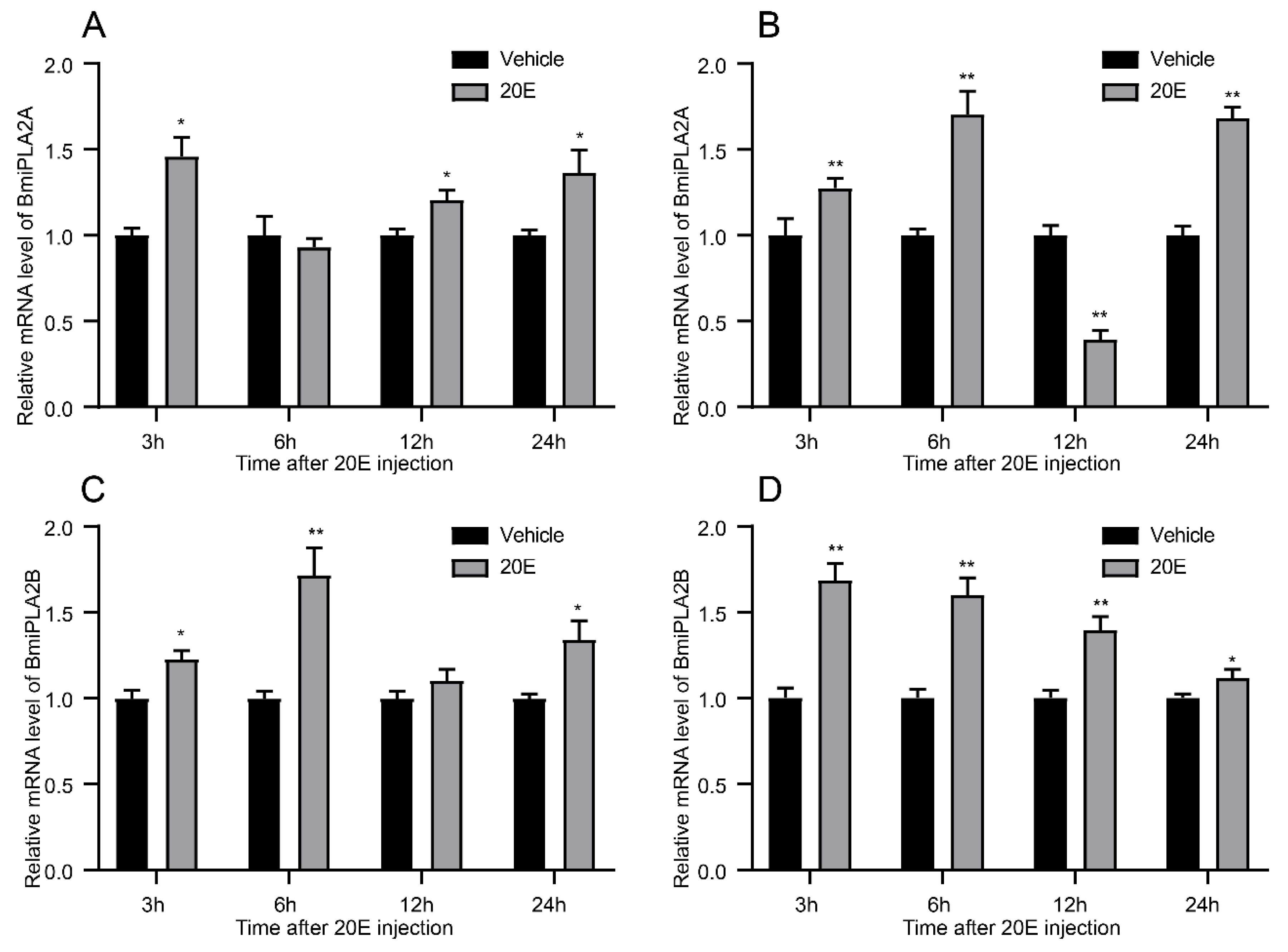
3.1.5. 20-Hydroxyecdysone Enhanced the Expression Levels of BmiPLA2s
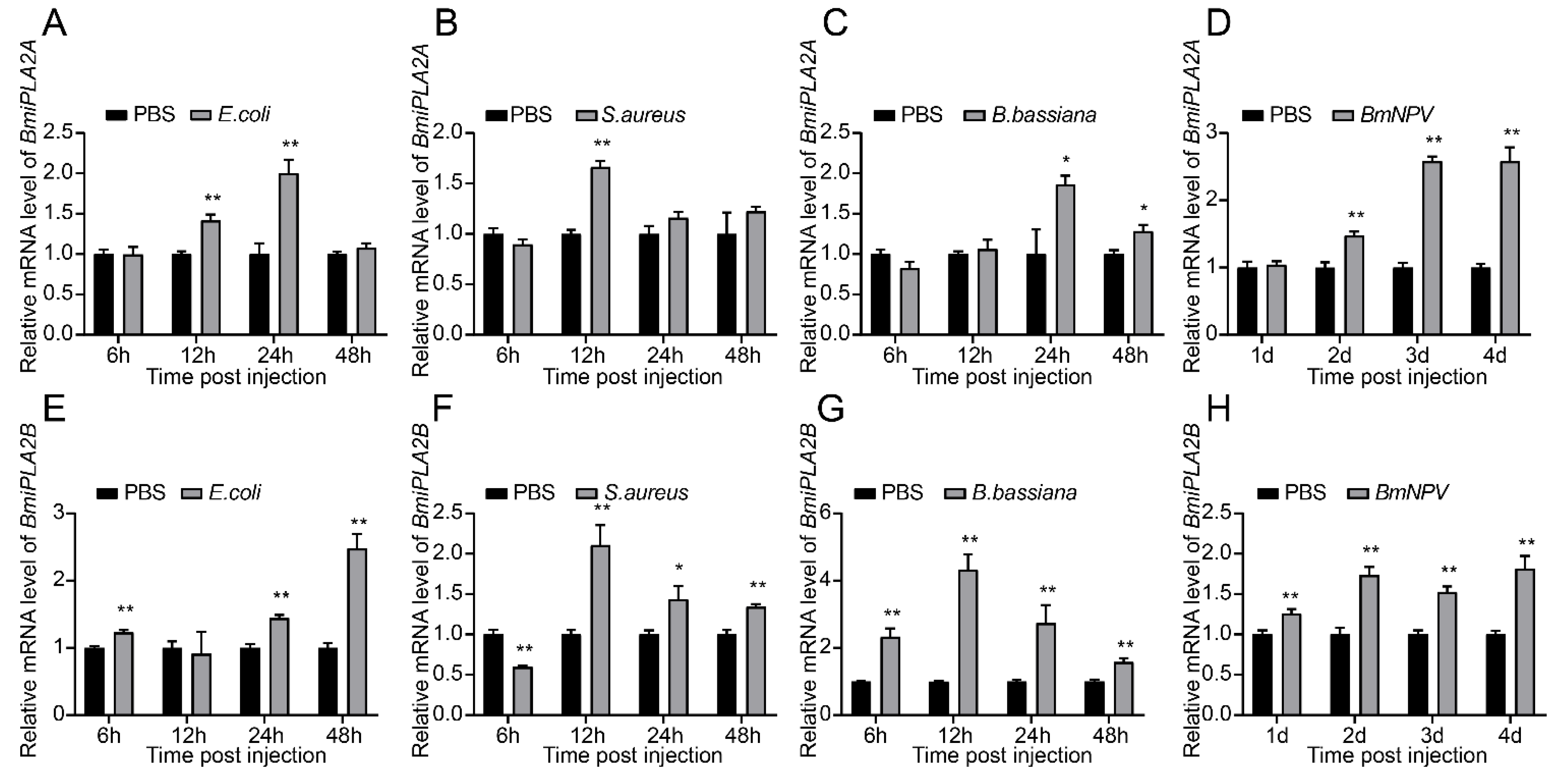
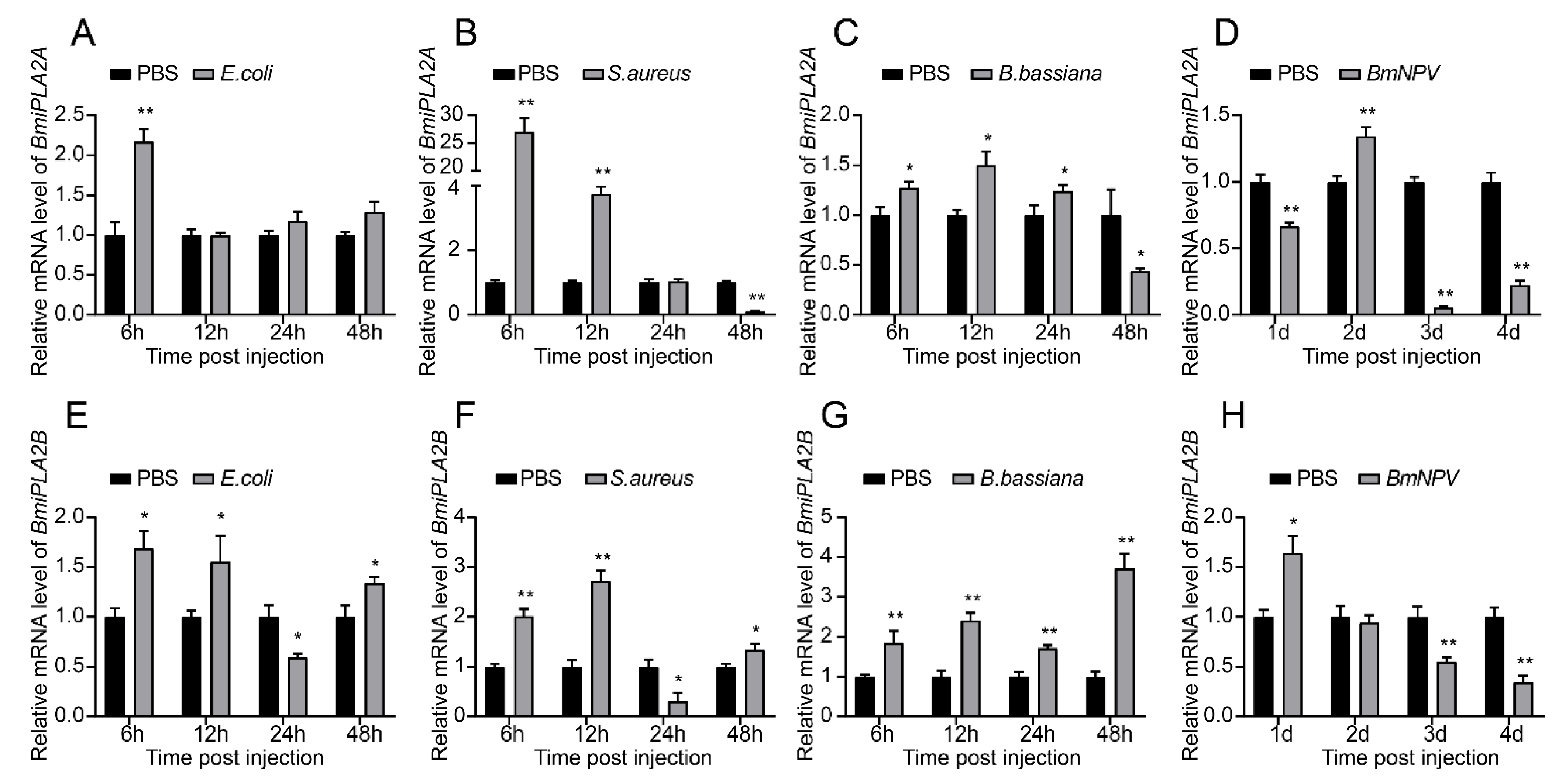
3.1.6. Responses of BmiPLA2s to Microbial Challenges
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vasquez, A.M.; Mouchlis, V.D.; Dennis, E.A. Review of four major distinct types of human phospholipase A2. Adv. Biol. Regul. 2017, 67, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2006, 1761, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Balsinde, J.; Balboa, M.A.; Dennis, E.A. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phos-pholipid fatty acid remodeling in murine P388D1 macrophages. J. Biol. Chem. 1997, 272, 29317–29321. [Google Scholar] [CrossRef] [Green Version]
- Rickard, A.; Portell, C.; Kell, P.J.; Vinson, S.M.; McHowat, J. Protease-activated receptor stimulation activates a Ca2+-independent phospholipase A2in bladder microvascular endothelial cells. Am. J. Physiol. Physiol. 2005, 288, F714–F721. [Google Scholar] [CrossRef]
- Song, K.; Zhang, X.; Zhao, C.; Ang, N.T.; Ma, Z.A. Inhibition of Ca2+-Independent Phospholipase A2 Results in Insufficient Insulin Secretion and Impaired Glucose Tolerance. Mol. Endocrinol. 2005, 19, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Kumar, S.; Kanumuri, R.; Stanley, D.; Kim, Y. A novel calcium-independent cellular PLA2 acts in insect immunity and larval growth. Insect Biochem. Mol. Biol. 2015, 66, 13–23. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Gautam, N.; Kim, Y. A novel calcium-independent phospholipase A2 and its physiological roles in de-velopment and immunity of a lepidopteran insect, Spodoptera exigua. Dev. Comp. Immunol. 2017, 77, 210–220. [Google Scholar] [CrossRef]
- Sajjadian, S.M.; Vatanparast, M.; Kim, Y. Toll/IMD signal pathways mediate cellular immune responses via induction of intracellular PLA 2 expression. Arch. Insect Biochem. Physiol. 2019, 101, e21559. [Google Scholar] [CrossRef]
- Iliadi, K.G.; Gluscencova, O.B.; Iliadi, N.; Boulianne, G.L. Mutations in the Drosophila homolog of human PLA2G6 give rise to age-dependent loss of psychomotor activity and neurodegeneration. Sci. Rep. 2018, 8, 2939. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.; Oh, Y.; Yoon, J.; Cho, W.; Baek, K. Molecular characterization of a gene encoding the Drosophila melanogaster phospholipase A2. Biochim. Biophys. Acta—Gene Struct. Expr. 2003, 1628, 206–210. [Google Scholar] [CrossRef]
- Vatanparast, M.; Ahmed, S.; Herrero, S.; Kim, Y. A non-venomous sPLA2 of a lepidopteran insect: Its physiological func-tions in development and immunity. Dev. Comp. Immunol. 2018, 89, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.B.M.; Lee, D.-W.; Jung, J.; Kim, Y. Deletion mutant of sPLA2 using CRISPR/Cas9 exhibits immunosuppression, developmental retardation, and failure of oocyte development in legume pod borer, Maruca vitrata. Dev. Comp. Immunol. 2019, 103, 103500. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, X.; Zheng, W.; Zhang, H. The PLA2 gene mediates the humoral immune responses in Bactrocera dorsalis (Hendel). Dev. Comp. Immunol. 2017, 67, 293–299. [Google Scholar] [CrossRef]
- Singh, C.O.; Xin, H.-H.; Chen, R.-T.; Wang, M.-X.; Liang, S.; Lu, Y.; Cai, Z.-Z.; Miao, Y.-G. BmPLA2 containing conserved domain WD40 affects the metabolic functions of fat body tissue in silkworm, Bombyx mori. Insect Sci. 2014, 23, 28–36. [Google Scholar] [CrossRef]
- Ji, J.Y.; Yin, Z.H.; Zhang, S.S.; Shen, D.X.; An, C.J. PLA2 mediates the innate immune response in Asian corn borer, Ostrinia furnacalis. Insect Sci. 2021. [Google Scholar] [CrossRef]
- Shrestha, S.; Park, Y.; Stanley, D.; Kim, Y. Genes encoding phospholipases A2 mediate insect nodulation reactions to bacterial challenge. J. Insect Physiol. 2010, 56, 324–332. [Google Scholar] [CrossRef]
- Figueiredo, M.B.; Genta, F.A.; Garcia, E.S.; Azambuja, P. Lipid mediators and vector infection: Trypanosoma rangeli inhibits Rhodnius prolixus hemocyte phagocytosis by modulation of phospholipase A2 and PAF-acetylhydrolase activities. J. Insect Physiol. 2008, 54, 1528–1537. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, M.; Su, J.; Yu, S.; Sun, Z.; Li, Y.; Zhang, W.; Hou, J.; Shang, L.; Cui, H. Characterization and identification of the integrin family in silkworm, Bombyx mori. Gene 2014, 549, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, K.; Pan, G.; Hao, X.; Li, C.; Li, C.; Gul, I.; Kausar, S.; Abbas, M.N.; Zhu, Y. The identification of nucle-ar factor Akirin with immune defense role in silkworm, Bombyx mori. Int. J. Biol. Macromol. 2021, 188, 32–42. [Google Scholar] [CrossRef]
- Zhang, K.; Tan, J.; Hao, X.; Tang, H.; Abbas, M.N.; Su, J.; Su, Y.; Cui, H. Bombyx mori U-shaped regulates the melanization cascade and immune response via binding with the Lozenge protein. Insect Sci. 2021. [Google Scholar] [CrossRef]
- Pan, G.; Zhang, K.; Li, C.; Hu, X.; Kausar, S.; Gu, H.; Yang, L.; Cui, H. A hemocyte-specific cathepsin L-like cysteine protease is involved in response to 20-hydroxyecdysone and microbial pathogens stimulation in silkworm, Bombyx mori. Mol. Immunol. 2020, 131, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tan, J.; Su, J.; Liang, H.; Shen, L.; Li, C.; Pan, G.; Yang, L.; Cui, H. Integrin β3 plays a novel role in innate im-munity in silkworm, Bombyx mori. Dev. Comp. Immunol. 2017, 77, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikos, F.; Clement, J.A.; Testa, E.; Ravindranathan, R.; Grey, C.; Dereli, I.; Bondarieva, A.; Valerio-Cabrera, S.; Stanzione, M.; Schleiffer, A.; et al. Mouse ANKRD31 Regulates Spatiotemporal Patterning of Meiotic Recombination Initiation and Ensures Recombination between X and Y Sex Chromosomes. Mol. Cell 2019, 74, 1069–1085.e11. [Google Scholar] [CrossRef]
- Park, Y.; Aliza, A.N.; Stanley, D. A secretory PLA2 associated with tobacco hornworm hemocyte membrane preparations acts in cellular immune reactions. Arch. Insect Biochem. Physiol. 2005, 60, 105–115. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Kwon, H.; Smith, R.C.; Sinnis, P. 20-Hydroxyecdysone Primes Innate Immune Responses That Limit Bacte-rial and Malarial Parasite Survival in Anopheles gambiae. MSPHERE 2020, 5, e00983-19. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Han, J.; Zhang, M.; Fan, J.; Gong, Q.; Ma, E.; Zhang, J. 20-Hydroxyecdysone enhances Immulectin-1 mediated immune response against entomogenous fungus in Locusta migratoria. Pest Manag. Sci. 2020, 76, 304–313. [Google Scholar] [CrossRef]
- Vatanparast, M.; Ahmed, S.; Sajjadian, S.M.; Kim, Y. A prophylactic role of a secretory PLA2 of Spodoptera exigua against entomopathogens. Dev. Comp. Immunol. 2019, 95, 108–117. [Google Scholar] [CrossRef]
- Han, G.D.; Na, J.; Chun, Y.S.; Kumar, S.; Kim, W.; Kim, Y. Chlorine dioxide enhances lipid peroxidation through inhibiting calcium-independent cellular PLA2 in larvae of the Indianmeal moth, Plodia interpunctella. Pestic. Biochem. Physiol. 2017, 143, 48–56. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef]
- Kim, H.; Choi, D.; Jung, J.; Kim, Y. Eicosanoid mediation of immune responses at early bacterial infection stage and its in-hibition by Photorhabdus temperatasubsp. temperata, an entomopathogenic bacterium. Arch. Insect Biochem. 2018, 99, e21502. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, X.; Feng, Q. Fat Body Biology in the Last Decade. Annu. Rev. Èntomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Èntomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
| Gene Name and Purpose | Primer Name | Annealing Temperature (°C) | Sequence (5’–3’) |
|---|---|---|---|
| BmiPLA2A cDNA cloning | BmiPLA2AF | 54.36 | ATGCTGAATTCATTTTTTCGTG |
| BmiPLA2AR | 56.74 | CTAACCGGTCGTGCCGTTGTGTTC | |
| BmiPLA2B cDNA cloning | BmiPLA2BF | 54.47 | AGCCAGGCTACTATACGGAGCG |
| BmiPLA2BR | 57.76 | TCAGTGCCCCTGGACTAGGCCCATG | |
| BmiPLA2A qPCR | iPLA2AF | 55.40 | ACTCATGCGGCGGTAAAGAA |
| iPLA2AR | 57.45 | TCTCCTTGTTGCTAGCTGCC | |
| BmiPLA2B qPCR | iPLA2BF | 57.45 | TCGGAGCCAAGTTGGAAGTC |
| iPLA2BR | 57.45 | CTGACCGCACCTTCCTTGAT | |
| Sw22934 qPCR | sw22934 F | 55.40 | TTCGTACTGGCTCTTCTCGT |
| sw22934 R | 51.95 | CAAAGTTGATAGCAATTCCCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhang, B.; Zheng, X.; Ji, H.; Feng, K.; Hu, X.; Gul, I.; Abbas, M.N.; Cui, H.; Zhu, Y. Molecular Characterization of Two Genes Encoding Novel Ca2+-Independent Phospholipase A2s from the Silkworm, Bombyx mori. Curr. Issues Mol. Biol. 2022, 44, 777-790. https://doi.org/10.3390/cimb44020054
Hu X, Zhang B, Zheng X, Ji H, Feng K, Hu X, Gul I, Abbas MN, Cui H, Zhu Y. Molecular Characterization of Two Genes Encoding Novel Ca2+-Independent Phospholipase A2s from the Silkworm, Bombyx mori. Current Issues in Molecular Biology. 2022; 44(2):777-790. https://doi.org/10.3390/cimb44020054
Chicago/Turabian StyleHu, Xin, Bili Zhang, Xi Zheng, Haoyan Ji, Kun Feng, Xiaosong Hu, Isma Gul, Muhammad Nadeem Abbas, Hongjuan Cui, and Yong Zhu. 2022. "Molecular Characterization of Two Genes Encoding Novel Ca2+-Independent Phospholipase A2s from the Silkworm, Bombyx mori" Current Issues in Molecular Biology 44, no. 2: 777-790. https://doi.org/10.3390/cimb44020054
APA StyleHu, X., Zhang, B., Zheng, X., Ji, H., Feng, K., Hu, X., Gul, I., Abbas, M. N., Cui, H., & Zhu, Y. (2022). Molecular Characterization of Two Genes Encoding Novel Ca2+-Independent Phospholipase A2s from the Silkworm, Bombyx mori. Current Issues in Molecular Biology, 44(2), 777-790. https://doi.org/10.3390/cimb44020054






