Abstract
Background/Objectives: Despite immune checkpoint inhibitors (ICPIs)’s transformation of lung cancer treatment, pneumonitis remains a potentially serious immune-related adverse event. However, reliable data on the comparative risks of individual ICPIs remain unknown. We conducted this network meta-analysis (NMA) to, therefore, quantify and compare the exact pooled burden of pneumonitis risk across multiple ICPI analogs. Methods: We searched the following databases, PubMed, Embase, Scopus MEDLINE and Cochrane Database of Systematic Reviews, as well as gray literature on Google Scholar for eligible studies reporting on the prevalence of pneumonitis following immune check point inhibitor exposures. Pairwise and network meta-analyses were performed to estimate pooled odds ratios (ORs) for pneumonitis, using placebo as the common comparator. Sensitivity analyses assessed the impact of study quality and combination therapies. Results: A total of 29 studies enrolling 15,271 patients with non-small cell lung cancer (NSCLC) or small-cell lung cancer (SCLC) satisfied the inclusion criteria and are included in the meta-analysis. Pembrolizumab was associated with a significantly increased risk of pneumonitis compared to placebo (odds ratio [OR] = 2.67, 95% confidence interval [CI]: 1.70–4.17), with similar elevated risk observed for sugemalimab (odds ratio [OR] = 2.45, 95% confidence interval [CI]: 1.52–3.95). Nivolumab was associated with increased odds of pneumonitis, although with unstable point estimate (odds Ratio [OR] = 2.69, 95% confidence interval [CI]: 0.64–11.35). Statistical heterogeneity was low (H statistics = 1.34). Atezolizumab and ipilimumab demonstrated modest or uncertain risk. Heterogeneity was low (I2 = 12%), and results were robust to sensitivity analyses. Higher pneumonitis rates were observed in combination regimens. Conclusions: Our analysis demonstrates that pneumonitis risk varies among ICPIs, with pembrolizumab and sugemalimab showing the highest odds. Although the absolute incidence is low, the potential severity of pneumonitis warrants vigilant monitoring. These results should guide clinicians in risk stratification and treatment planning, and they should support the development of standardized reporting criteria and further comparative research.
1. Introduction
Immune checkpoint inhibitors (ICPIs) have significantly improved clinical outcomes in patients with non-small cell lung cancer (NSCLC) and small-cell lung cancer [1,2,3,4]. They inhibit immunologic checkpoints, thereby restoring T-cell activation and antitumor immunity in patients with cancer [1]. Pembrolizumab and nivolumab are monoclonal antibodies that block the interaction between programmed cell death 1 and its ligands, PD-L1 and PD-L2 [1], whereas atezolizumab, sugemalimab, and durvalumab are monoclonal antibodies that bind programmed death ligand 1, blocking its engagement with PD-1. Ipilimumab is a monoclonal antibody that binds cytotoxic T-lymphocyte antigen-4, blocking its interaction with CD80 and CD86 [1]. Despite this significant improvement in clinical outcomes with the advent of these agents, immune-related adverse events (irAEs) such as pneumonitis continue to pose serious clinical challenges. Pneumonitis, while rare, can lead to significant morbidity and mortality, particularly in patients with pre-existing pulmonary comorbidities or those receiving combination therapies [1]. The aggregate of current evidence suggests varying pneumonitis risks across different ICPIs, yet direct comparisons remain limited. Previous pairwise meta-analyses and pharmacovigilance reports have indicated potential differences in toxicity profiles between PD-1 and PD-L1 inhibitors [2]. However, these analyses often lack the scope and statistical rigor of network-based approaches, especially when those approaches are based not only on the broad adverse effects burden of ICPIs but rather on specific morbidities such as pneumonitis [5]. Emerging nanotechnology-based therapies, including nanoparticle-mediated drug delivery systems, are being explored to improve the therapeutic index of lung cancer treatments by enhancing tumor targeting and reducing systemic toxicity [6]. These innovations underscore a broader need for treatment platforms that maintain anticancer efficacy without the substantial immune-mediated toxicities—such as pneumonitis—currently observed with ICPIs.
Reported pneumonitis incidence varies widely across trials and observational datasets due to differences in diagnostic criteria, radiologic confirmation, grading approaches, and attribution practices [7]. Long-term experience with nivolumab, for example, demonstrates delayed presentation of pneumonitis but also highlights inconsistencies in how it is monitored and reported across studies [4]. More recent first-line immunotherapy trials, including CheckMate-227, illustrate substantial heterogeneity in the capture of pulmonary toxicities and variation in the thresholds used to classify and confirm pneumonitis-related events [8]. Retrospective studies add further limitations, including small cohort sizes and incomplete imaging review, as well as diagnostic uncertainty when distinguishing pneumonitis from infection, tumor progression, or radiation-induced lung injury [9,10]. Mortality estimates for severe pneumonitis remain imprecise; findings, such as the high mortality reported by Tone et al., are derived from single-center experiences subject to referral and selection biases [11]. Evidence guiding rechallenge is likewise constrained, as safety data predominantly reflect highly selected patients fit for retreatment after grade ≥ 2 irAEs [12]. Broader irAE registries also exhibit reporting heterogeneity, with variable inclusion of low-grade or subclinical cases and inconsistent clinician-reported grading [2]. Related toxicities such as ICPI-associated myocarditis further demonstrate the spectrum of immune-mediated injury, yet mechanistic insight and biomarker-based risk stratification remain limited [13]. These collective limitations underscore the need for more standardized, prospective, and mechanistically grounded characterization of ICPI-related pneumonitis to enhance early detection and clinical management.
Immune checkpoint inhibitor-associated pneumonitis (ICI-pneumonitis) represents one of the most clinically significant immune-related adverse events of immunotherapy [9]. Although relatively uncommon, occurring in approximately 2–5% of treated patients, it carries disproportionate morbidity and mortality [7,10]. Severe (grade ≥ 3) cases occur in about 0.8%, frequently necessitating immunotherapy discontinuation, hospitalization, and high-dose corticosteroid therapy [11,12,13]. Fatal outcomes are reported in up to 30% of severe cases [14,15]. The burden extends beyond patient outcomes, as ICI-pneumonitis often mimics infection, radiation injury [16], or tumor progression, creating diagnostic uncertainty and delaying cancer therapy. Additionally, real-world data show higher incidence rates compared with clinical trials [17], likely to reflect broader patient comorbidities and combined ICI regimens. Given the expanding use of ICIs across malignancies, awareness, early detection, and standardized management are crucial to mitigate this growing clinical and healthcare burden. Despite this enormous burden and ever-present uncertainty regarding the type of patients who are likely to develop immune-related ICPI complications, outcomes from primary studies have been conflicting. Even subsequent pooled analyses, such as the meta-analytical synthesis by Su et al. [18], still left a lot of unresolved concerns regarding the exact numerical comparative pneumonitis burden of various ICPI analogs in patients in these cohorts of patients. Although other factors, such as performance in RCT as well as affordability, will rank higher on the factors that determine the choice of individual ICPIs, having additional information on the comparative risks of IMAE (such as pneumonitis) will additionally support this attempt at risk stratification. Furthermore, since the publication of the last meta-analysis, additional studies have been published, further providing additional data points for the comparative determination of risk [4,5,8,12].
In this network meta-analysis, we have carried out comparative pooled risk assessment on pneumonitis through both direct and indirect comparison between various ICPIs (such as pembrolizumab). This is with the view to determining the exact pneumonitis burden of each and, in this way, guide therapeutic choices. This study addresses limitations of prior reviews by including a broader set of studies and applying sensitivity analyses to assess the robustness of our resulting point estimates (across study designs, treatment contexts, and patient subgroups).
2. Materials and Methods
The systematic review protocol was registered in the International PROSPERO database (International Prospective Register of Systematic Reviews; https://www.crd.york.ac.uk/PROSPERO/view/CRD420251079154 (assessed on 23 June 2025)) under the registration number CRD420251079154. Registration was completed prior to data collection and analysis. All methods and inclusion criteria were implemented as initially planned. This is systematic review and network meta-analysis was conducted in compliance with the PRISMA guidelines.
2.1. Search Strategy
A comprehensive literature search was undertaken to identify randomized controlled trials evaluating pneumonitis risk associated with immune checkpoint inhibitors (ICPIs) in patients with lung cancer. The search was conducted across multiple electronic databases, including PubMed, Embase, Scopus, MEDLINE, and the Cochrane Database of Systematic Reviews, from database inception to June 2025. In addition, gray literature sources, such as Google Scholar and trial registries (ClinicalTrials.gov and WHO ICTRP), were reviewed to ensure completeness and minimize publication bias. The search combined Medical Subject Headings (MeSH) and free-text terms relating to the interventions and outcome of interest. The final search syntax was structured as follows: (“immune checkpoint inhibitors” OR “PD-1” OR “PD-L1” OR “CTLA-4”) AND (“pneumonitis” OR “interstitial lung disease” OR “lung toxicity”) AND (“randomized controlled trial”) AND (“lung cancer” OR “SCLC” OR “NSCLC”). The reference lists of all included articles and relevant review papers were manually screened to identify additional eligible studies.
2.2. Eligibility Criteria
Eligible studies were randomized controlled trials that enrolled adult patients (≥18 years) with histologically confirmed non-small cell lung cancer (NSCLC) or small-cell lung cancer (SCLC) who received treatment with at least one immune checkpoint inhibitor targeting PD-1, PD-L1, or CTLA-4 pathways, either as monotherapy or in combination with other agents. Studies were required to report extractable data on the incidence or number of pneumonitis cases of any grade, as defined by Common Terminology Criteria for Adverse Events (CTCAE). Trials that compared ICPIs with placebo, standard chemotherapy, or other ICPIs were included. Exclusion criteria were preclinical or non-oncologic studies, trials enrolling pediatric populations, studies involving non-randomized or single-arm designs, and those that did not provide sufficient data to calculate pneumonitis event rates or effect estimates.
2.3. Study Selection and Data Extraction
All retrieved citations were imported into the Rayyan QCRI software (Rayyan QCRI; Qatar Computing Research Institute, Hamad Bin Khalifa University, Doha, Qatar) for reference management and duplicate removal. Two reviewers (RE and MID) independently screened titles and abstracts to identify potentially eligible studies. The full texts of all potentially relevant articles were then obtained and reviewed in detail. Disagreements between reviewers regarding inclusion or exclusion were resolved by consensus, and if unresolved, arbitration was undertaken by a third reviewer (AA).
A standardized data extraction form was developed and piloted on ten randomly selected studies to ensure uniformity and reliability. The extracted information included details on study design, publication year, country, phase, sample size, histologic subtype, intervention type, comparator arm, ICPI dosage and regimen, follow-up duration, and the number of pneumonitis cases (any grade and grade ≥ 3, where available). When essential data were missing or unclear, corresponding authors were contacted to obtain clarification.
2.4. Risk of Bias Assessment
The methodological quality of the included studies was evaluated using the Cochrane Risk of Bias 2.0 tool, and the results were visualized using the ROBVIS web application (developed by Evidence Synthesis Ireland and the Centre for Open Science, Galway, Ireland and Bristol, UK). This tool assesses the internal validity of randomized trials across six core domains: the randomization process, deviations from intended interventions, completeness of outcome data, accuracy of outcome measurement, selective reporting, and other potential sources of bias. Each domain was rated as low, some concerns, or high risk of bias. Two reviewers (AA and MID) performed independent assessments, and discrepancies were resolved through discussion. Studies classified as having a high risk of bias were subjected to sensitivity analyses to determine their influence on pooled estimates.
2.5. Network Geometry and Treatment Nodes
A network plot was constructed to illustrate the geometry of treatment comparisons among the included trials. Each node represented a distinct intervention, and edges denoted direct head-to-head comparisons between treatments. The node size was weighted according to the number of participants who received that particular treatment, while the thickness of each connecting line reflected the number of trials contributing to that comparison. Placebo served as the reference node, forming the largest and most interconnected point within the network. The network comprised seven active ICPIs: pembrolizumab, nivolumab, durvalumab, atezolizumab, avelumab, sugemalimab, and ipilimumab. The geometry revealed a well-connected, star-shaped structure centered on placebo, ensuring the feasibility of indirect comparisons across agents. There were no disconnected components or closed loops that would threaten the transitivity assumption required for network meta-analysis.
2.6. Statistical Analysis
All analyses adhered to PRISMA guidelines for network meta-analyses. The primary outcome was the odds ratio (OR) for any-grade pneumonitis associated with ICPI therapy. Initially, pairwise meta-analyses were performed for comparisons between each ICPI and placebo using a random-effects DerSimonian–Laird model to account for between-study heterogeneity. Heterogeneity was quantified using the I2, Q, and H statistics, with an I2 value exceeding 50% indicating substantial heterogeneity.
Subsequently, a frequentist random-effects network meta-analysis (NMA) was undertaken to estimate pooled odds ratios and 95% confidence intervals using placebo as the common comparator. The relative ranking of each intervention was determined using P-scores, which estimate the probability that a given treatment is among the most effective or least harmful options. The assumption of transitivity was verified by ensuring comparability of clinical and methodological characteristics across trials.
Consistency between direct and indirect evidence was examined using the design-by-treatment interaction model and node-splitting approaches, with p-values > 0.05 indicating satisfactory global and local consistency, respectively. Usually, network forest plots are generated based on Bayesian methods that rank treatments by the surface under the cumulative ranking curve (usually called SUCRA). From a frequentist perspective (which was our methodology), treatment effects are thought of as fixed parameters and thus, strictly speaking, a concept like SUCRA does not apply. A frequentist alternative called the P-score was proposed, but SUCRA or P-scores have no major advantage compared to ranking treatments by their point estimates [19]. Rather, treatments are ranked by their effect sizes in the network forest plot, with a dot in the plot giving the central estimate, and the line representing the confidence interval. Sensitivity analyses were conducted to evaluate the robustness of results, excluding studies with a high risk of bias and those evaluating combination regimens, thereby isolating the effects of monotherapy. Additional subgroup analyses were stratified by cancer type (NSCLC versus SCLC), ICPI class (PD-1 versus PD-L1 versus CTLA-4 inhibitors), and trial phase. Publication bias was assessed through visual inspection of funnel plot symmetry and confirmed using Egger’s regression test. All analyses were carried out in MetaXL (version 5.3, EpiGear International, Queensland, Australia), R (netmeta package, R Core Team, Vienna, Austria; https://www.R-project.org, accessed on 5 December 2025), and Stata version 15 (metan and network commands; StataCorp LLC, College Station, TX, USA.
3. Results
3.1. Study Characteristics
Table 1 shows the full socio-demographic characteristics of the included studies. Electronic search of relevant databases returned n = 965 titles and abstracts, from which n = 29 studies were eligible for inclusion into the systematic review and network meta-analysis (Figure 1). A total of 29 studies [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] were included in this network meta-analysis, encompassing patients primarily with non-small cell lung cancer (NSCLC), 82.76%, and a minority with small-cell lung cancer (SCLC), 17.2%. The majority of the studies were conducted in early-phase settings: Phase I (27.6%) [25,26,34,36,37,45], Phase II (62.1%) [20,21,22,23,24,30,31,32,33,35,39,42,43,46,47,48], and Phase III (10.3%) [28,38,39].

Table 1.
Socio-demographic characteristics as well as the interventions and proportion of adverse effect in the included studies. The sample sizes across trials varied widely, ranging from as few as 13 participants [21] to over 10,000 [28,41], reflecting both exploratory and confirmatory trial designs. The median age of participants across studies ranged from 50 to 72 years, with most cohorts being predominantly male. Smoking history, although clinically relevant in pneumonitis risk, was inconsistently reported across the included studies.
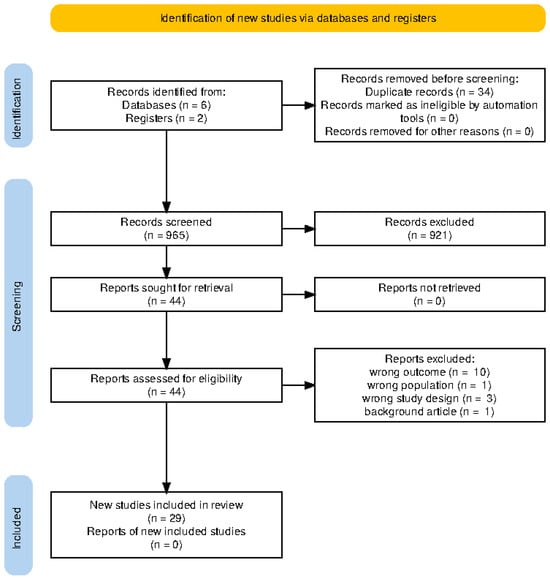
Figure 1.
Total number of studies retrieved from the initial search of relevant registries and databases, as well as the final number of studies included in the review following exhaustive screening.
3.2. Prevalence Estimates Stratified by Type of ICPI
Regarding the types of immune checkpoint inhibitors (ICPIs) explored, pembrolizumab was the most frequently studied (40.7%), followed by nivolumab (22.2%), atezolizumab (14.8%), ipilimumab (11.1%), durvalumab (7.4%), and sugemalimab (3.7%), as shown in Table 1. Combination regimens, involving concurrent chemotherapy, radiotherapy, or dual ICPI therapy, reflect real-world treatment strategies and clinical trial complexity. The Common Terminology Criteria for Adverse Events (CTCAE) was used for adverse event grading in nearly all studies, although a few used modified criteria or did not specify the grading approach.
3.3. Pairwise Meta-Analysis (Pembrolizumab vs. Placebo)
To estimate the risk of pneumonitis with pembrolizumab relative to placebo, a pairwise meta-analysis was conducted using 10 studies directly comparing these interventions. The pooled odds ratio was 2.67 (95% confidence interval [CI]: 1.70 to 4.17), indicating that pembrolizumab was associated with a statistically significant increase in the risk of pneumonitis (Figure 2). This analysis incorporated a broad range of study populations and treatment settings, including both monotherapy and combination regimens. Additional comparison between other ICPIs and placebo is shown in the Appendix A (Scheme A2).
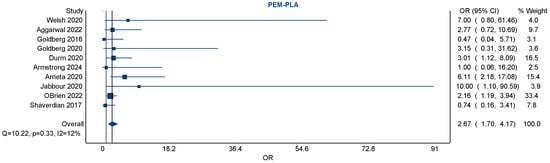
Figure 2.
A forest plot of pairwise meta-analyses of studies exploring the risk of pneumonitis amongst NSCLC patients exposed to pembrolizumab vs. placebo (usual standard of care). There was negligible heterogeneity amongst the ten included studies (with an I2 of 12%). Pembrolizumab was associated with a significantly increased risk of pneumonitis, with a pooled odds ratio of 2.67 [95% CI 1.70–4.17] [20,23,24,25,34,35,41,42,43,47].
The heterogeneity among studies was low, with an I2 statistic of 12%, a Q-statistic of 10.22 (p = 0.33), and an H-statistic of 1.06, suggesting good consistency and minimal between-study variance. The relatively narrow confidence interval, along with the low heterogeneity, supports the reliability of this finding across multiple trials.
3.4. Small Study Effect and Publication Bias
Visual inspection of the forest plot showed that although individual study effect estimates varied, they consistently favored an increased risk with pembrolizumab over placebo. A notable contributor to the weight of the analysis was the large RCT (O’Brien 2022) [41], which alone accounted for over 33% of the pooled estimate, demonstrating the influence of large trials in driving overall findings. Smaller studies contributed less weight but did not significantly deviate from the overall trend.
3.5. Network Meta-Analysis (NMA)
A comprehensive network meta-analysis was conducted to compare the pneumonitis risk across all ICPIs, incorporating both direct and indirect comparisons. The network plot revealed a highly connected network centered on placebo, which was compared directly or indirectly with nearly every other agent. This strong connectivity bolstered the statistical robustness of the indirect comparisons, as in Figure 3.
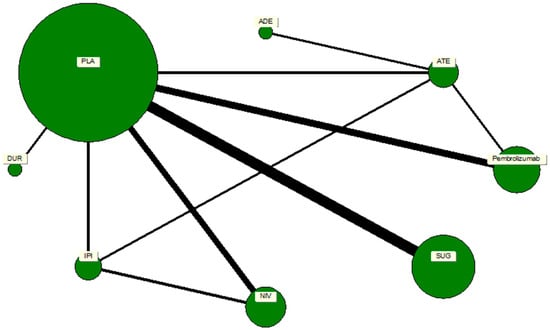
Figure 3.
A network map of direct and indirect comparisons of the immune checkpoint inhibitor analogs. The size of the nodes depicts the number of studies associated with a particular drug or treatment regimen, whereas the thickness of the connecting strands indicates the number of studies associated between the two drugs.
Direct effect estimates from the NMA highlighted pembrolizumab, nivolumab, and sugemalimab as being associated with higher odds of pneumonitis relative to placebo. Specifically, pembrolizumab had an odds ratio of 2.67 (95% confidence interval [CI]: 1.70 to 4.17), nivolumab had an odds ratio of 2.69 (95% confidence interval [CI]: 0.64 to 11.35), and sugemalimab had an odds ratio of 2.45 (95% confidence interval [CI]: 1.52 to 3.95). These findings suggest that these agents pose a notably elevated risk for pneumonitis. However, confidence intervals for nivolumab were wider due to fewer direct comparisons, indicating less certainty in the point estimate, as in Table 2.

Table 2.
Showing point estimates of both direct and indirect comparison between various immune checkpoint inhibitors and placebo, as well as indirect comparison between various ICPI analogs.
Other agents showed more modest or uncertain effects. Ipilimumab demonstrated an odds ratio of 1.93 (95% confidence interval [CI]: 0.06 to 59.87), while atezolizumab showed an odds ratio of 1.33 (95% confidence interval [CI]: 0.38 to 4.62). These results suggest potential but non-significant increases in risk, complicated by wide confidence intervals reflecting sparse data or high variance (all of which suggest instability of the point estimate). The indirect estimates comparing each ICPI to pembrolizumab revealed that sugemalimab had a comparable risk (odds ratio [OR] = 0.92, 95% confidence interval [CI]: 0.48 to 1.77), while atezolizumab was associated with a potentially lower risk (odds ratio [OR] = 0.50, 95% confidence interval [CI]: 0.13 to 1.87). Other agents, such as ipilimumab and durvalumab, demonstrated even lower odds compared to pembrolizumab, although these estimates were accompanied by extremely wide confidence intervals, limiting interpretability. Despite this, the consistency in the directionality of effect estimates suggests a potential hierarchy of pneumonitis risk, with pembrolizumab and sugemalimab at the upper end, as in Table 2.
3.6. Sensitivity Analyses
Sensitivity analyses were performed to assess the robustness of the primary findings. Exclusion of studies with a high risk of bias had minimal impact on the pooled estimates, with the odds ratio for pembrolizumab vs. placebo decreasing slightly but remaining statistically significant, suggesting that the observed association was not driven by study quality alone. Additional sensitivity analyses were conducted to account for the impact of combination therapies. When studies employing concurrent chemo-radiotherapy or dual ICPI regimens were excluded, the pooled odds ratio for pembrolizumab decreased modestly from 2.67 to approximately 2.1. This attenuation suggests that combination regimens may contribute modestly to pneumonitis risk, but that pembrolizumab independently confers an elevated risk.
Subgroup analyses by cancer type (NSCLC vs. SCLC) and by trial phase (Phase III vs. earlier) revealed broadly similar patterns of risk, though statistical power was limited due to small numbers in some strata. Overall, the direction and magnitude of effect remained consistent across sensitivity analyses, lending confidence to the main findings.
3.7. Heterogeneity and Publication Bias
The assessment of heterogeneity in the pairwise meta-analysis revealed low between-study variance, supported by an I2 of 12%, Q = 10.22 (p = 0.33), and H = 1.06. These values indicate that variation in effect sizes was likely due to chance rather than true heterogeneity. Visual inspection of the funnel plot for pembrolizumab vs. placebo showed general symmetry, providing no strong evidence of small study effects or publication bias, as in Figure 4.
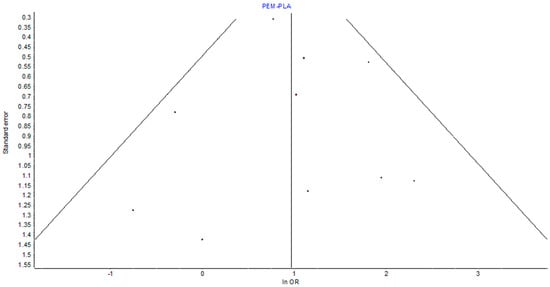
Figure 4.
Funnel plot showing the effect of bias due to small studies. It shows the symmetry of the distribution of studies around the unity point (OR 1). This confirms the lack of significant small study effects on the overall point estimates.
3.8. Pneumonitis Outcomes and Adverse Event Reporting
Across studies, the incidence of grade ≥ 3 pneumonitis ranged from 0% to as high as 7% in the pembrolizumab arms, confirming that pneumonitis, though infrequent, can be clinically significant and occasionally life-threatening. All but four studies [30,37,42,44] used CTCAE criteria for grading adverse events, promoting consistency in reporting across the dataset. Notably, combination regimens tended to report higher rates of pneumonitis than monotherapy studies, underscoring the importance of evaluating treatment context. These findings are relevant for clinical decision-making in tailoring treatment based on patient risk profiles and expected toxicity burden.
3.9. Risk of Bias Assessment Outcomes
Assessment of study quality revealed that 81.5% of studies were considered at high risk of bias, primarily due to open-label design, small sample sizes, or limited pneumonitis reporting. Only one large-scale RCT (Liu et al., 2023 [28]) was deemed to have a low risk of bias, while two other studies were rated as having some concerns, as shown in Figure 5.
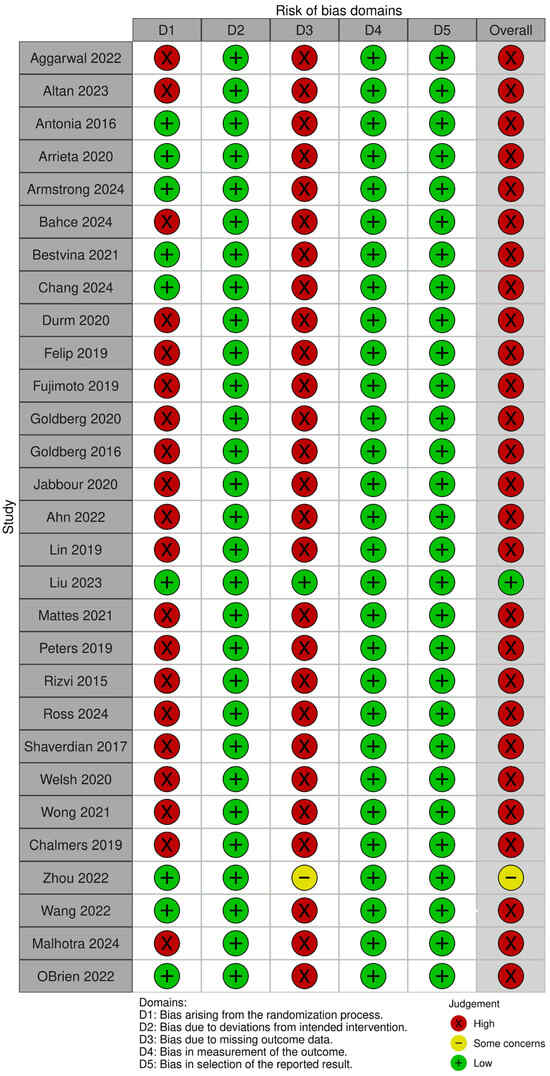
Figure 5.
A pictorial representation of the risk of bias as a marker of methodological quality of the included studies across 5 domains. The green, red, and yellow color codes of the overall outcomes denote low, high, and moderate risks of bias, respectively [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
4. Discussion
In this network meta-analysis of RCTs, we found increased pneumonitis risk associated with pembrolizumab compared to other ICPTs. This network meta-analysis provides a nuanced evaluation of pneumonitis risk across different immune checkpoint inhibitors (ICPIs) in lung cancer treatment, highlighting pembrolizumab and sugemalimab as having the highest relative risks. These findings build upon prior meta-analyses while offering new insights enabled by network-level comparisons and robust sensitivity testing. The increased pneumonitis risk associated with pembrolizumab (odds ratio [OR] = 2.67, 95% confidence interval [CI]: 1.70–4.17) aligns with several previous meta-analyses and pharmacovigilance studies. For example, a systematic review by Wang et al. [39]. found that PD-1 inhibitors, particularly pembrolizumab, were associated with a higher incidence of immune-related pneumonitis compared to PD-L1 inhibitors, potentially due to broader immune activation by PD-1 blockade. Our findings extend this knowledge by confirming the elevated risk through both direct and indirect comparisons across a larger and more heterogeneous patient population.
In contrast, the point estimate for nivolumab (odds ratio [OR] = 2.69) was also elevated but accompanied by a wide confidence interval (95% confidence interval [CI]: 0.64–11.35), reflecting imprecise estimation due to fewer direct comparisons. A similar trend was noted in the work of Ramalingham et al. [5], who observed that nivolumab carried a non-trivial pneumonitis risk, albeit slightly lower than pembrolizumab. Sugemalimab, a newer PD-L1 inhibitor, demonstrated a high odds ratio (odds ratio [OR] = 2.45, 95% confidence interval [CI]: 1.52–3.95), which may appear contradictory to the general perception that PD-L1 inhibitors carry a lower pneumonitis risk. However, its elevated risk in our analysis might be attributed to limited real-world safety data, smaller sample sizes, and the inclusion of high-risk populations in clinical trials. Additionally, sugemalimab trials have been conducted predominantly in Asian populations, and the existing literature suggests that pneumonitis rates may vary by ethnicity, with higher susceptibility reported among East Asian patients [5].
Atezolizumab and ipilimumab showed lower and less consistent associations with pneumonitis. Atezolizumab’s relatively favorable toxicity profile has been supported by prior pharmacovigilance reports and meta-analyses [33]. Ipilimumab, an anti-CTLA-4 antibody, has a distinct mechanism of action that may result in different immune-related adverse event patterns, with colitis and dermatitis often more prominent than pneumonitis [5,21].
Socio-demographic factors, though not consistently reported across studies, are important to consider in interpreting pneumonitis risk. Age is a recognized risk factor; older patients may have decreased pulmonary reserve. Males were overrepresented, and smoking status was inconsistently documented, both of which could influence observed toxicity rates.
These findings underscore the need for personalized risk–benefit assessments in ICPI therapy selection and for standardized adverse event reporting in future trials.
The pattern observed in our analysis, where pembrolizumab and sugemalimab demonstrated the highest odds of pneumonitis (OR 2.67 and OR 2.45, respectively), with nivolumab showing a similarly elevated but imprecise estimate, aligns with the underlying biological differences between PD-1 and PD-L1 blockade [49]. PD-1 inhibitors, such as pembrolizumab and nivolumab, exert broader immunologic disruption by simultaneously blocking PD-1 interactions with both PD-L1 and PD-L2 [49]. This dual inhibition is particularly relevant in the lung, where PD-L2 expression on airway epithelial and dendritic cells contributes to maintaining local immune tolerance and limiting excessive T-cell-mediated inflammation [50]. Interference with PD-1/PD-L2 signaling has been associated with enhanced Th1 and Th17 responses, increased interferon-γ activity, and greater susceptibility to alveolar injury—mechanistic pathways that plausibly explain the higher pneumonitis risk observed with PD-1 agents in our study [49,50]. In contrast, PD-L1 inhibitors, such as sugemalimab, block PD-L1 while preserving PD-1/PD-L2 signaling, theoretically conferring a more restricted immune activation profile [51,52]. The elevated pneumonitis risk we observed with sugemalimab suggests that PD-L1 blockade can still meaningfully perturb pulmonary immune homeostasis, particularly in patients with lung cancer, where background inflammation, tumor burden, or prior thoracic irradiation may amplify susceptibility. The modest or uncertain risk estimates seen with atezolizumab and ipilimumab are consistent with this graded biological model, and the higher pneumonitis rates in combination regimens reinforce the additive effect of intensified immune activation. Overall, our findings reflect and support the mechanistic hierarchy of pulmonary toxicity across ICPI classes, underscoring the need for vigilant monitoring, individualized risk assessment, and standardized pneumonitis-reporting frameworks.
In our network meta-analysis, each drug was individually evaluated not on the basis of its mechanism of action but as an individual analog of ICPI. Nonetheless, the point estimate of the burden of individual ICPIs as a representative class (for example, peembro as PD-1) that we found aligns with what is reported in the literature [8,11]. Monoclonal antibodies that bind programmed death ligand 1, blocking its engagement with PD-1, including atezolizumab, sugemalimab, and durvalumab, are associated with varying risks of pneumonitis. For example, atezolizumab had an odds ratio of 1.33 (95% confidence interval [CI]: 0.38 to 4.62), durvalumab had an odds ratio of 2.06 (95% [CI]: 0.17 to 23.88), and sugemalimab had an odds ratio [OR] of 2.45 (95% confidence interval [CI]: 1.52–3.95). Conversely, monoclonal antibody drugs that block the interaction between programmed cell death 1 and its ligands, PD-L1 and PD-L2 (including pembrolizumab and nivolumab), are associated with varying risks of pneumonitis [odds ratio [OR] of 2.67 (95% confidence interval [CI]: 1.70–4.17) and odds ratio [OR] of 2.69 (95% confidence interval [CI]: 0.64–11.35)], respectively. In general, PD-1 inhibitors were associated with a comparatively higher incidence of immune-related pneumonitis compared to PD-L1 inhibitors and the anti-CTLA-4 antibody, while the latter showed lower and less consistent associations with pneumonitis.
Several recent meta-analyses have sought to clarify the determinants of ICPI-related pneumonitis, yet each is limited by methodological constraints that temper the certainty of its conclusions [53,54,55]. Zhou et al. (2025) [55] synthesized risk factors for pneumonitis among lung cancer patients receiving immunotherapy; however, the studies contributing to their pooled estimates displayed considerable variability in pneumonitis definitions, imaging confirmation, and clinical attribution practices [55]. Many lacked multidisciplinary review or standardized grading, raising concerns regarding outcome misclassification and generating uncertainty around their reported point estimates. Li et al. (2024) [53] examined COPD as a potential modifier of pneumonitis risk, concluding that pre-existing lung disease confers higher susceptibility. However, the underlying evidence base was predominantly retrospective, with uneven reporting of pulmonary function metrics, emphysema burden, smoking exposure, and prior thoracic irradiation—all factors that may independently influence pneumonitis risk. Consequently, the observed association between COPD and CIP remains susceptible to residual confounding [53]. In contrast, our study addresses several of these recurring limitations by applying a rigorously defined pneumonitis outcome incorporating radiologic and clinical criteria, using standardized grading and adjudication procedures, and integrating more granular covariates reflective of real-world clinical complexity. This approach allows a more precise delineation of risk and provides a clearer framework for interpreting pneumonitis within contemporary immunotherapy practice.
The risk of bias assessment (Figure 5) showed that most included randomized trials had a high risk of bias in at least one domain, with several demonstrating concerns across D1 (randomization process) and D3 (missing outcome data). These domains are critical for outcome validity determination, especially in safety analyses where pneumonitis events are relatively infrequent and susceptible to detection and reporting variability. High risk in the randomization process raises the possibility of baseline imbalances or allocation bias, which could inflate or obscure treatment-related adverse events. Similarly, missing outcome data—often due to loss to follow-up or treatment discontinuation—may disproportionately affect pneumonitis ascertainment, thereby reducing confidence in the effect estimates. Despite these limitations, domains relating to the measurement of outcomes (D4) and selection of the reported result (D5) were generally adjudicated as low risk, reflecting consistent and protocol-driven reporting of pneumonitis across the trials
Our findings have several direct translational implications for both clinicians and regulatory agencies. Firstly, the observation that pembrolizumab and sugemalimab confer the highest pneumonitis risk, and with consistent directionality for nivolumab, provides an evidence-based foundation for potential risk-informed treatment selection. For oncologists, these results support the need for stricter pulmonary surveillance protocols, earlier radiologic assessment in symptomatic or high-risk patients, and heightened vigilance when initiating PD-1 or PD-L1 inhibitors in individuals with baseline lung morbidities or prior radiation therapy. Second, the low heterogeneity (I2 = 12%) and robustness of our sensitivity analyses strengthen the generalizability of these risk estimates across real-world practice, offering regulators a potentially more stable signal when evaluating post-marketing safety, updating product labels, or issuing risk-mitigation recommendations. Third, the consistently higher pneumonitis rates observed in combination regimens underscore the importance of adjusting trial monitoring requirements and mandating clearer attribution frameworks where radiotherapy, chemotherapy, and immunotherapy overlap. Collectively, the relevance of these findings lies in enabling more precise risk stratification, guiding surveillance intensity, and refining regulatory decision-making around the safe deployment of immune checkpoint inhibitors.
Strengths and Limitations
Strengths of this study include the comprehensive scope of the network meta-analysis, which allowed both direct and indirect comparisons of multiple ICPIs. Previous network meta-analytical attempts have focused on the general immune-mediated adverse effects of ICPIs, potentially diluting the requisite rigor required for reporting the exact burden of this adverse effect. The novelty of our approach is the laser focus on pneumonitis, which, therefore, provides an exact comparative point estimate of its risk in this cohort of patients. The inclusion of monotherapy and combination therapy regimens reflects real-world practice. Robust sensitivity analyses, low heterogeneity, and symmetrical funnel plots further support the credibility of our findings.
However, the aforementioned strength analysis of these data schemes is fraught with lots of limitations. This includes the predominance of early-phase studies and variable pneumonitis definitions. Sparse data for some agents led to wide confidence intervals, and socio-demographic data were often missing or inconsistently reported, limiting detailed subgroup analysis. Additionally, the Common Terminology Criteria for Adverse Events (CTCAE) were used for adverse event grading in nearly all studies. One study used modified criteria, specifically the Immune-related Response Criteria and Common Terminology Criteria (IRE and CTCAE) for Adverse Events, while four studies did not specify the grading approach. This, however, did not significantly impact the final point estimate of the comparative difference on the burden of pneumonitis among ICPI analogs. Additional factors, such as differences in patient population and age disparities, though not explored in our current review, are likely to account for some of the heterogeneity seen on the various point estimates apparent in our meta-analysis.
These factors may account for some of the observed high heterogeneity of our study outcome. Nevertheless, the stability of our point estimates across both direct and indirect comparisons suggests that these limitations are unlikely to significantly impact the burden of pneumonitis vis-à-vis ICPI exposure.
5. Conclusions
Our analysis demonstrates that pneumonitis risk varies among ICPIs, with pembrolizumab and sugemalimab showing the highest odds. Although the absolute incidence is low, the potential severity of pneumonitis warrants vigilant monitoring. These results should guide clinicians in risk stratification and treatment planning, and support the development of standardized reporting criteria and further comparative research.
Author Contributions
M.I.D. was involved in review concept development, the literature search, screening, risk of bias assessment, data analyses, result interpretation, and the writing of the initial draft and final manuscript, and was the guarantor of the review. R.A.R.E. was involved in the literature search, study registration on Prospero, screening, data curation, independent review, risks of bias review, result interpretation, and writing of initial and final manuscript drafts. I.Y.A. was involved in data screening, data curation, independent review, risks of bias review, and writing of initial and final manuscript drafts. A.A. was involved in data screening validation, data curation, independent review, risks of bias review, and the writing of initial and final manuscript drafts. All authors have read and agreed to the published version of the manuscript.
Funding
Funding of the article processing charge of this article was provided by Medical Research Center (MRC) Hamad Medical Corporation, Doha Qatar.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Ruba Abdulrazaq Ebzee, Ahmed Aboughalia and Mohammed I. Danjuma were employed by the Hamad Medical Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. None of the authors have any conflict of interest to declare with regard to this manuscript.
Appendix A
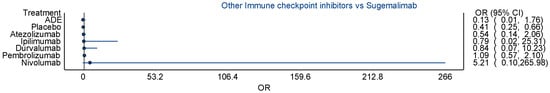
Scheme A1.
Figure showing comparative estimate between sugemalimab and other ICPIs.
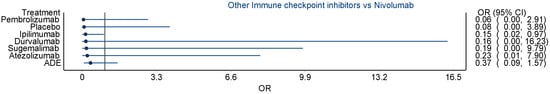
Scheme A2.
Figure showing comparative estimate between nivolumab and other ICPIs.
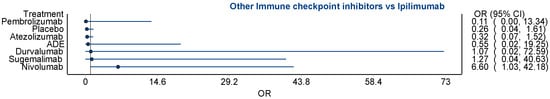
Scheme A3.
Figure showing comparative estimate between ipilimumab and other ICPIs.
References
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., III; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; Volume 94. [Google Scholar]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2015, 33, 2004–2012. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Schenker, M.; Caro, R.B.; Lee, K.H.; Zurawski, B.; Audigier-Valette, C.; Provencio, M.; et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1. J. Clin. Oncol. 2025, 38, 9500. [Google Scholar] [CrossRef]
- Girigoswami, A.; Girigoswami, K. Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment. Genes 2023, 14, 1370. [Google Scholar] [CrossRef]
- George, S.; Bell, E.J.; Zheng, Y.; Kim, R.; White, J.; Devgan, G.; Smith, J.; Lal, L.S.; Engel-Nitz, N.M.; Liu, F.X. The Impact of Adverse Events on Health Care Resource Utilization, Costs, and Mortality Among Patients Treated with Immune Checkpoint Inhibitors. Oncologist 2021, 26, e1205–e1215. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Hryniewicki, A.T.; Wang, C.; Shatsky, R.A.; Coyne, C.J. Management of Immune Checkpoint Inhibitor Toxicities: A Review and Clinical Guideline for Emergency Physicians. J. Emerg. Med. 2018, 55, 489–502. [Google Scholar] [CrossRef]
- Yan, C.; Huang, M.; Swetlik, C.; Toljan, K.; Mahadeen, A.Z.; Bena, J.; Kunchok, A.; Funchain, P.; McGinley, M. Predictors for the development of neurological immune-related adverse events of immune checkpoint inhibitors and impact on mortality. Eur. J. Neurol. 2023, 30, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Tone, M.; Izumo, T.; Awano, N.; Kuse, N.; Inomata, M.; Jo, T.; Yoshimura, H.; Minami, J.; Takada, K.; Miyamoto, S.; et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac. Cancer 2019, 10, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Allouchery, M.; Lombard, T.; Martin, M.; Rouby, F.; Sassier, M.; Bertin, C.; Atzenhoffer, M.; Miremont-Salame, G.; Perault-Pochat, M.-C.; Puyade, M. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J. Immunother. Cancer 2020, 8, e001622. [Google Scholar] [CrossRef]
- Champion, S.N.; Stone, J.R. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod. Pathol. 2020, 33, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Koks, M.S.; Ocak, G.; Suelmann, B.B.M.; Hulsbergen-Veelken, C.A.R.; Haitjema, S.; Vianen, M.E.; Verhaar, M.C.; Kaasjager, K.A.H.; Khairoun, M. Immune checkpoint inhibitor-associated acute kidney injury and mortality: An observational study. PLoS ONE 2021, 16, e0252978. [Google Scholar] [CrossRef]
- Reddy, H.; Weis, T.; Hough, S.; Daignault-Newton, S.; Schneider, B. P2.04-35 Immune Related Adverse Events in NSCLC Patients Treated with Immune Checkpoint Therapy Who Received the Influenza Vaccination vs No Vaccination. J. Thorac. Oncol. 2019, 14, S722. [Google Scholar] [CrossRef]
- Ahmed, K.A.; Grass, G.D.; Creelan, B.; Gray, J.; Kim, S.; Dilling, T.J.; Antonia, S.; Perez, B. Tolerability and Safety of Thoracic Radiation and Immune Checkpoint Inhibitors Among Patients with Lung Cancer. Int. J. Radiat. Oncol. 2017, 98, 224. [Google Scholar] [CrossRef]
- Lippenszky, L.; Mittendorf, K.F.; Kiss, Z.; LeNoue-Newton, M.L.; Napan-Molina, P.; Rahman, P.; Ye, C.; Laczi, B.; Csernai, E.; Jain, N.M.; et al. Prediction of Effectiveness and Toxicities of Immune Checkpoint Inhibitors Using Real-World Patient Data. JCO Clin. Cancer Inform. 2024, 8, e2300207. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, E.C.; Wu, J.B.; Li, T.; Hou, Y.L.; Wang, D.Y.; Gao, Z.H. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: A systematic review and meta-analysis. Front. Immunol. 2019, 10, 108. [Google Scholar] [CrossRef]
- Doi, S.A.R.; Barendregt, J.J. A generalized pairwise modelling framework for network meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 187–194. [Google Scholar] [CrossRef]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; Zandberg, D.P.; et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: Interim results from a multicenter phase I/II trial. J. Immunother. Cancer 2022, 10, e004424. [Google Scholar] [CrossRef] [PubMed]
- Altan, M.; Wang, Y.; Song, J.; Welsh, J.; Tang, C.; Guha-Thakurta, N.; Blumenschein, G.R.; Carter, B.W.; Wefel, J.S.; Ghia, A.J.; et al. Nivolumab and ipilimumab with concurrent stereotactic radiosurgery for intracranial metastases from non-small cell lung cancer: Analysis of the safety cohort for non-randomized, open-label, phase I/II trial. J. Immunother. Cancer 2023, 11, e006871. [Google Scholar]
- Fujimoto, D.; Yomota, M.; Sekine, A.; Morita, M.; Morimoto, T.; Hosomi, Y.; Ogura, T.; Tomioka, H.; Tomii, K. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: A multicenter, open-label single-arm phase II trial. Lung Cancer 2019, 134, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Schalper, K.A.; Gettinger, S.N.; Mahajan, A.; Herbst, R.S.; Chiang, A.C.; Lilenbaum, R.; Wilson, F.H.; Omay, S.B.; Yu, J.B.; et al. Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 655–663. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef]
- Jabbour, S.K.; Berman, A.T.; Decker, R.H.; Lin, Y.; Feigenberg, S.J.; Gettinger, S.N.; Aggarwal, C.; Langer, C.J.; Simone, C.B., 2nd; Bradley, J.D.; et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol. 2020, 6, 848–855. [Google Scholar] [CrossRef]
- Ahn, M.J.; Cho, B.C.; Ou, X.; Walding, A.; Dymond, A.W.; Ren, S.; Cantarini, M.; Jänne, P.A. Osimertinib Plus Durvalumab in Patients With EGFR-Mutated, Advanced NSCLC: A Phase 1b, Open-Label, Multicenter Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 718–723. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, Y.; Yao, L.; Kalhor, N.; Carter, B.W.; Altan, M.; Blumenschein, G.; Byers, L.A.; Fossella, F.; Gibbons, D.L.; et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J. Thorac. Oncol. Off Publ. Int. Assoc. Study Lung Cancer 2020, 15, 248–257. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, C.; Huang, Y.; Huang, R.; Huang, S.M.; Larkins, E.; Stapleford, L.; Rivera, D.R.; Kluetz, P.G.; Wang, S.; et al. Evaluating Pneumonitis Incidence in Patients with Non–small Cell Lung Cancer Treated with Immunotherapy and/or Chemotherapy Using Real-world and Clinical Trial Data. Cancer Res. Commun. 2023, 3, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Mattes, M.D.; Eubank, T.D.; Almubarak, M.; Wen, S.; Marano, G.D.; Jacobson, G.M.; Ma, P.C. A Prospective Trial Evaluating the Safety and Systemic Response From the Concurrent Use of Radiation Therapy with Checkpoint Inhibitor Immunotherapy in Metastatic Non–Small Cell Lung Cancer. Clin. Lung Cancer 2021, 22, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Felip, E.; Dafni, U.; Belka, C.; Guckenberger, M.; Irigoyen, A.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—The ETOP NICOLAS trial. Lung Cancer 2019, 133, 83–87. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Mazières, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef]
- Ross, H.J.; Kozono, D.; Wang, X.F.; Urbanic, J.J.; Williams, T.M.; Nelson, G.D.; Carbone, D.P.; Chung, D.; Robb, R.; Byun, W.Y.; et al. Atezolizumab before and after Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase II Nonrandomized Controlled Trial. JAMA Oncol. 2024, 10, 1212–1219. [Google Scholar]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [PubMed]
- Welsh, J.W.; Heymach, J.V.; Guo, C.; Menon, H.; Klein, K.; Cushman, T.R.; Verma, V.; Hess, K.R.; Shroff, G.; Tang, C.; et al. Phase 1/2 Trial of Pembrolizumab and Concurrent Chemoradiation Therapy for Limited-Stage SCLC. J. Thorac. Oncol. 2020, 15, 1919–1927. [Google Scholar] [CrossRef]
- Wong, D.J.; Bauer, T.M.; Gordon, M.S.; Bene-Tchaleu, F.; Zhu, J.; Zhang, X.; Cha, E. Safety and Clinical Activity of Atezolizumab Plus Ipilimumab in Locally Advanced or Metastatic Non–Small Cell Lung Cancer: Results From a Phase 1b Trial. Clin. Lung Cancer 2022, 23, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, A.W.; Patel, S.; Boucher, K.; Cannon, L.; Esplin, M.; Luckart, J.; Graves, N.; Van Duren, T.; Akerley, W. Phase I Trial of Targeted EGFR or ALK Therapy with Ipilimumab in Metastatic NSCLC with Long-Term Follow-Up. Target. Oncol. 2019, 14, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, M.; Jiang, O.; Pan, Y.; Hu, D.; Lin, Q.; Wu, G.; Cui, J.; Chang, J.; Cheng, Y.; et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): Interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 209–219. [Google Scholar]
- Wang, J.; Zhou, C.; Yao, W.; Wang, Q.; Min, X.; Chen, G.; Xu, X.; Li, X.; Xu, F.; Fang, Y.; et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 739–747. [Google Scholar]
- Malhotra, J.; Chiappori, A.; Fujioka, N.; Hanna, N.H.; Feldman, L.E.; Patel, M.; Moore, D.; Chen, C.; Jabbour, S.K. Phase I/II trial of plinabulin in combination with nivolumab and ipilimumab in patients with recurrent small cell lung cancer (SCLC): Big ten cancer research consortium (BTCRC-LUN17-127) study. Lung Cancer 2024, 195, 107932. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Arrieta, O.; Barrón, F.; Ramírez-Tirado, L.A.; Zatarain-Barrón, Z.L.; Cardona, A.F.; Díaz-García, D.; Yamamoto Ramos, M.; Mota-Vega, B.; Carmona, A.; Peralta Álvarez, M.P.; et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller, W.H.; Hansen, A.R.; Lee, J.-S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: A phase 2 randomized trial. Investig. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef]
- Bahce, I.; Dickhoff, C.; Schneiders, F.L.; Veltman, J.; Heineman, D.J.; Hashemi, S.M.S.; Vrijmoet, A.; Houda, I.; Ulas, E.B.; Bakker, J.; et al. Single-arm trial of neoadjuvant ipilimumab plus nivolumab with chemoradiotherapy in patients with resectable and borderline resectable lung cancer: The INCREASE study. J. Immunother. Cancer 2024, 12, e009799. [Google Scholar] [CrossRef]
- Bestvina, C.M.; Pointer, K.B.; Karrison, T.; Al-Hallaq, H.; Hoffman, P.C.; Jelinek, M.J.; Juloori, A.; Melotek, J.M.; Murgu, S.; Partouche, J.; et al. A Phase 1 Trial of Concurrent or Sequential Ipilimumab, Nivolumab, and Stereotactic Body Radiotherapy in Patients With Stage IV NSCLC Study. J. Thorac. Oncol. 2022, 17, 130–140. [Google Scholar] [CrossRef]
- Chang, J.Y.; Lin, S.H.; Dong, W.; Liao, Z.; Gandhi, S.J.; Gay, C.M.; Zhang, J.; Chun, S.G.; Elamin, Y.Y.; Fossella, F.V.; et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet 2023, 402, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Durm, G.A.; Jabbour, S.K.; Althouse, S.K.; Liu, Z.; Sadiq, A.A.; Zon, R.T.; Jalal, S.I.; Kloecker, G.H.; Williamson, M.J.; Reckamp, K.L.; et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non–small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer 2020, 126, 4353–4361. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Ardizzoni, A.; Ciuleanu, T.; Cobo, M.; Laktionov, K.; Szilasi, M.; Califano, R.; Carcereny, E.; Griffiths, R.; Paz-Ares, L.; et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur. J. Cancer 2020, 127, 160–172. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Sun, Y.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Chang, J.; Chen, J.; et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): Interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022, 23, 220–233. [Google Scholar] [CrossRef]
- Dhillon, S.; Duggan, S. Sugemalimab: First Approval. Drugs 2022, 82, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zheng, L.; Xu, X.; Jin, J.; Li, X.; Zhou, L. The impact of chronic obstructive pulmonary disease on the risk of immune-related pneumonitis in lung cancer patients undergoing immunotherapy: A systematic review and meta-analysis. BMC Pulm. Med. 2024, 24, 393. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Y.; Ying, Y.; Chen, R.; Wang, Z.; Lv, X. Risk factors for checkpoint inhibitor pneumonitis in lung cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2025, 16, 1607170. [Google Scholar] [CrossRef] [PubMed]
- Villacampa, G.; Cresta Morgado, P.; Carità, L.; Navarro, V.; Pascual, T.; Dienstmann, R. Safety and efficacy of antibody-drug conjugates plus immunotherapy in solid tumours: A systematic review and meta-analysis. Cancer Treat. Rev. 2024, 131, 102847. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).