Covalently Functionalized Halloysite-Calixarene Nanotubes for Injectable Hydrogels: A Multicavity Platform for Hydrophobic Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
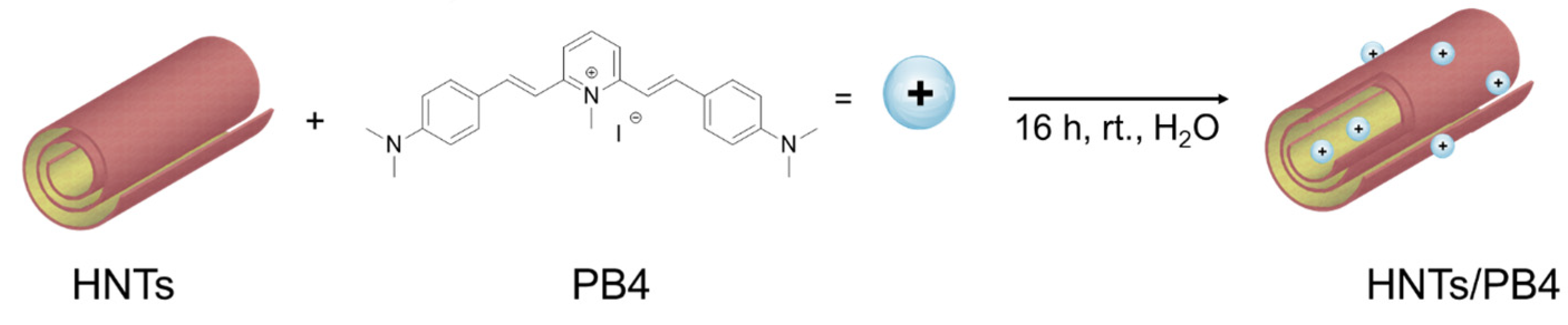
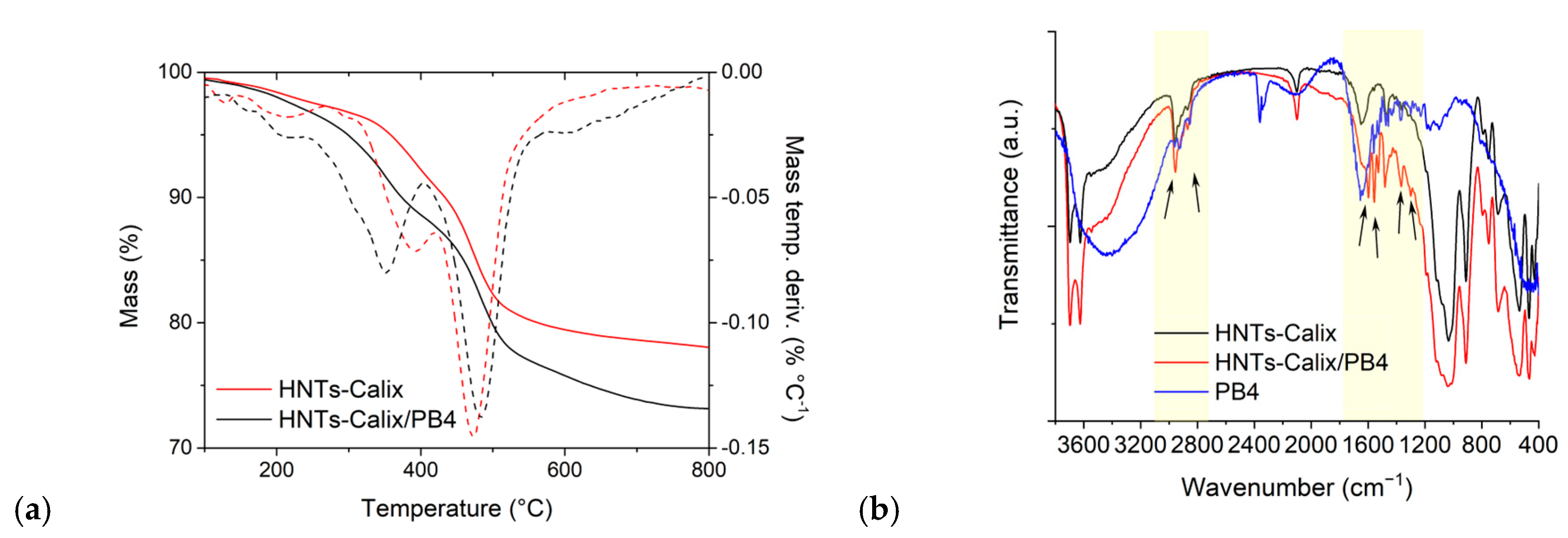
2.1. Synthesis of HNTs/PB4 Nanomaterial
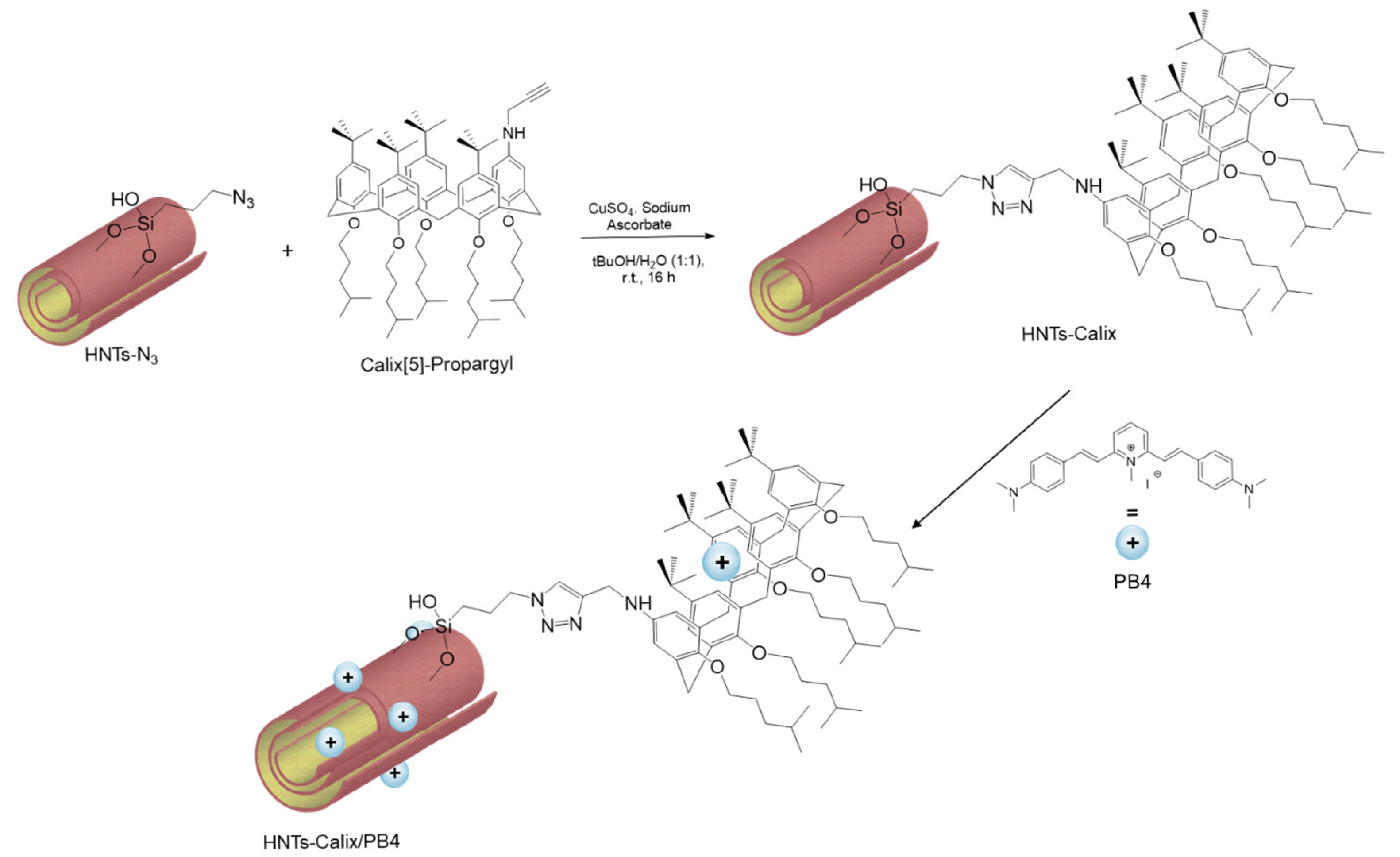
2.2. Synthesis of HNTs-Calix/PB4 Nanomaterial
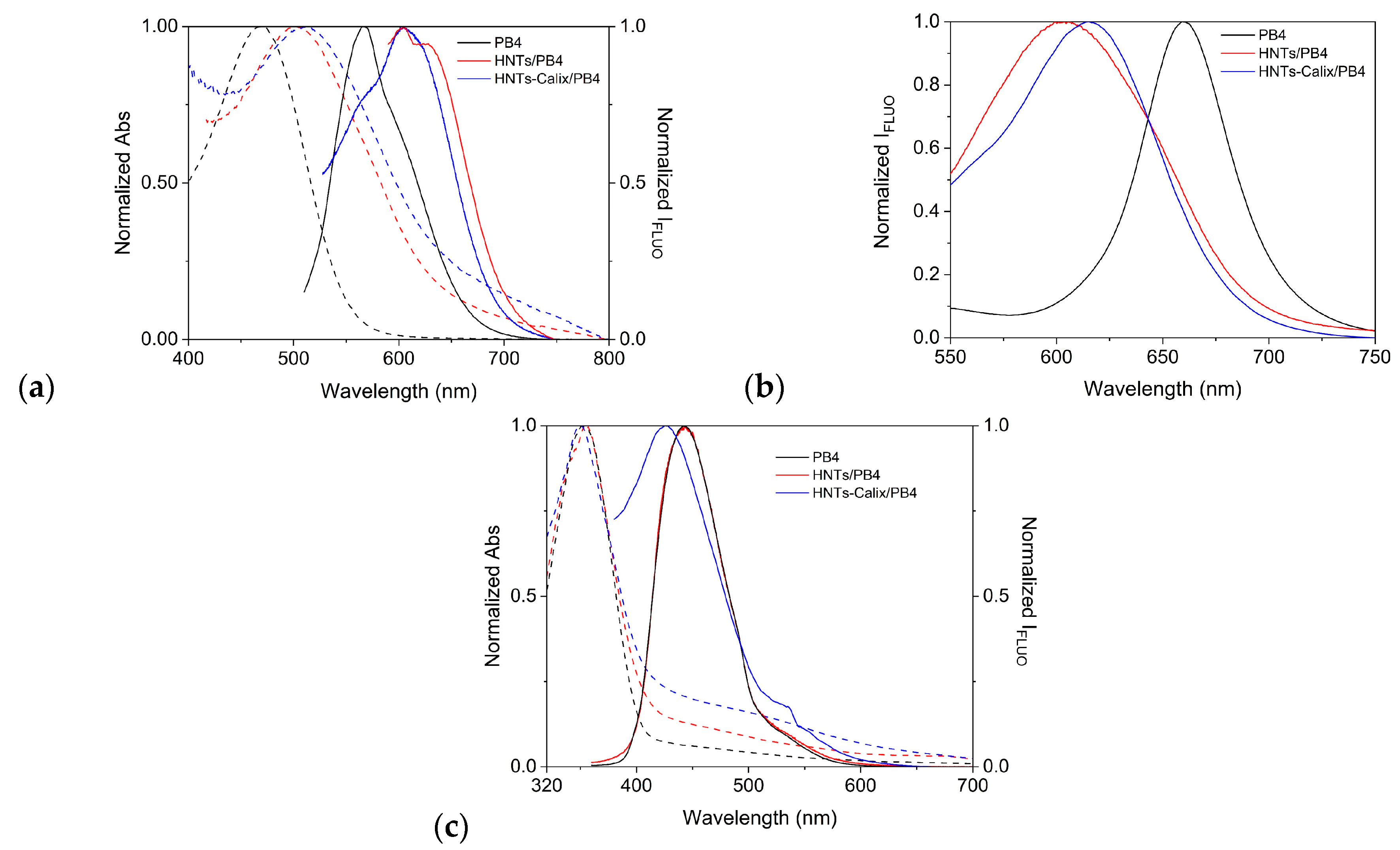
2.3. Photophysical Properties of HNTs/PB4 and HNTs-Calix/PB4 Nanomaterials
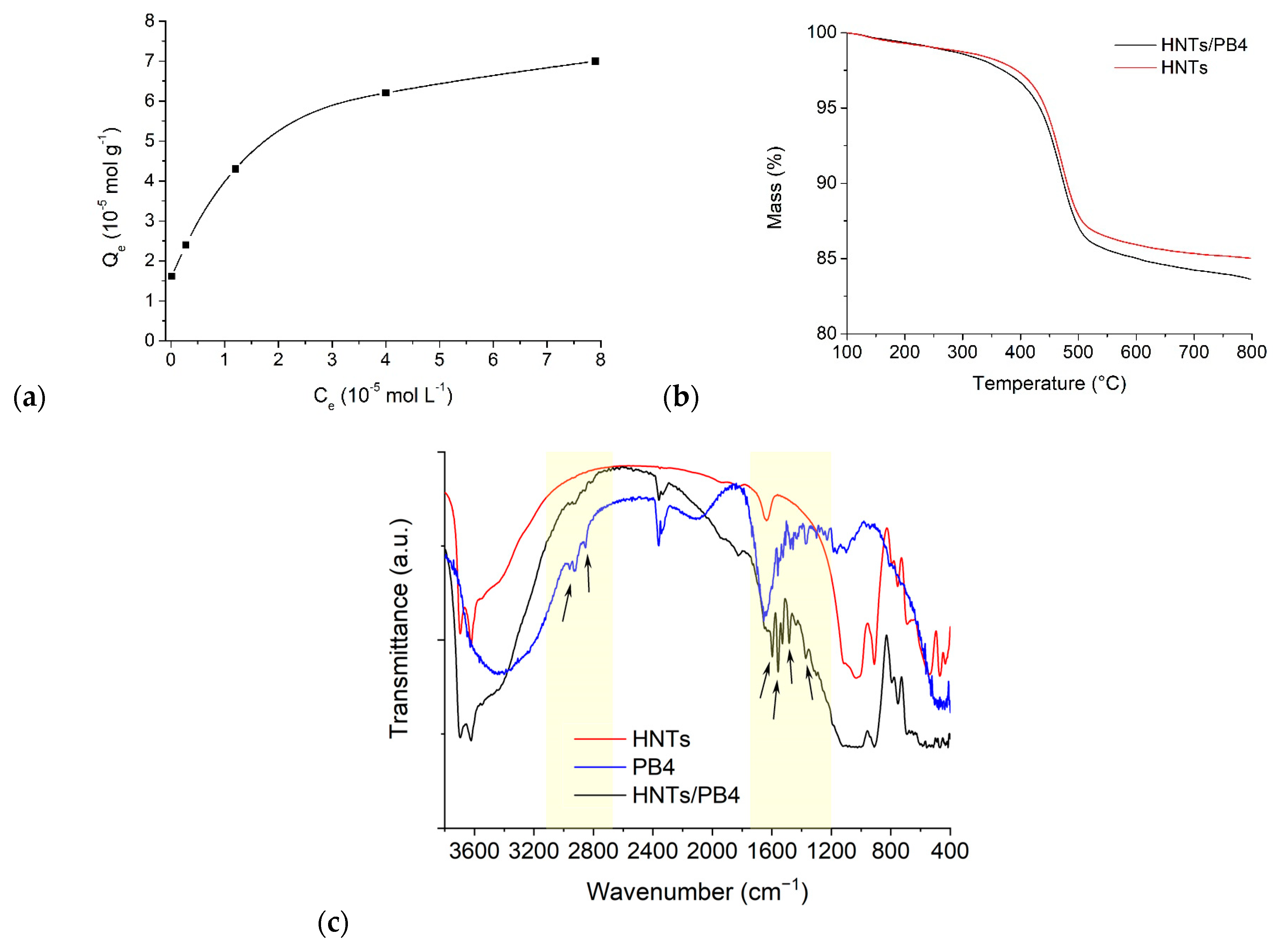
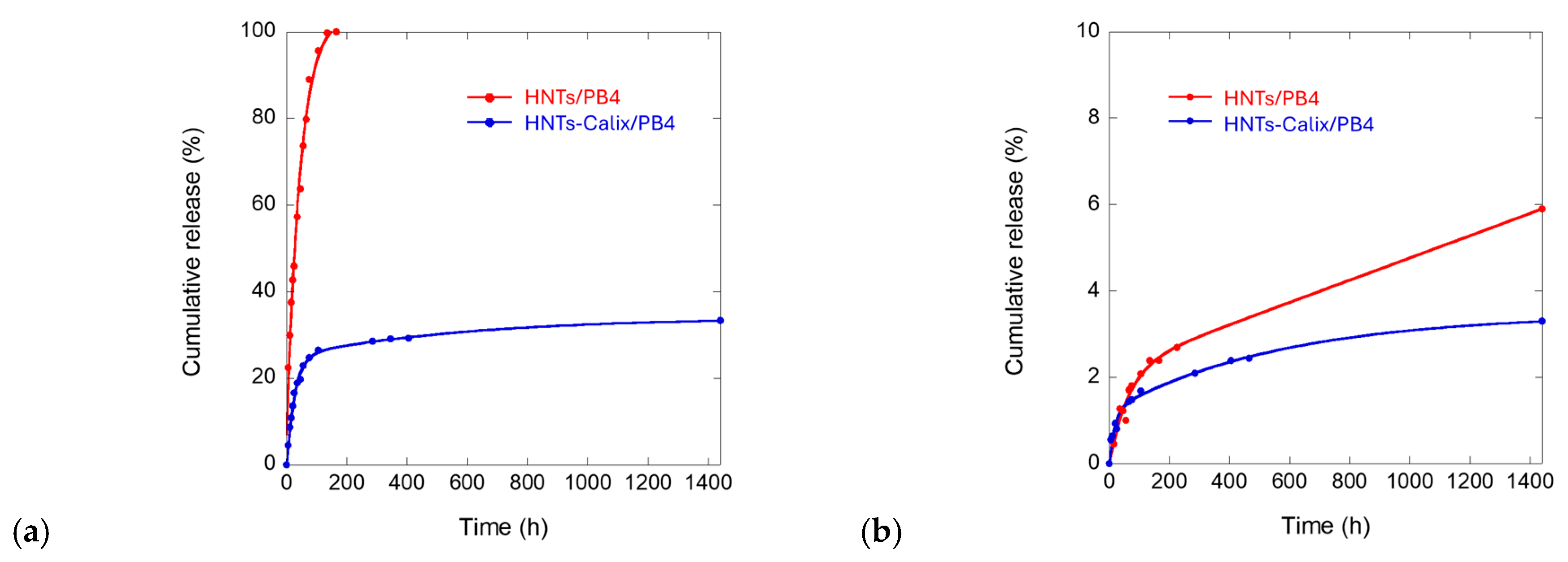
2.4. Kinetic Release Experiments
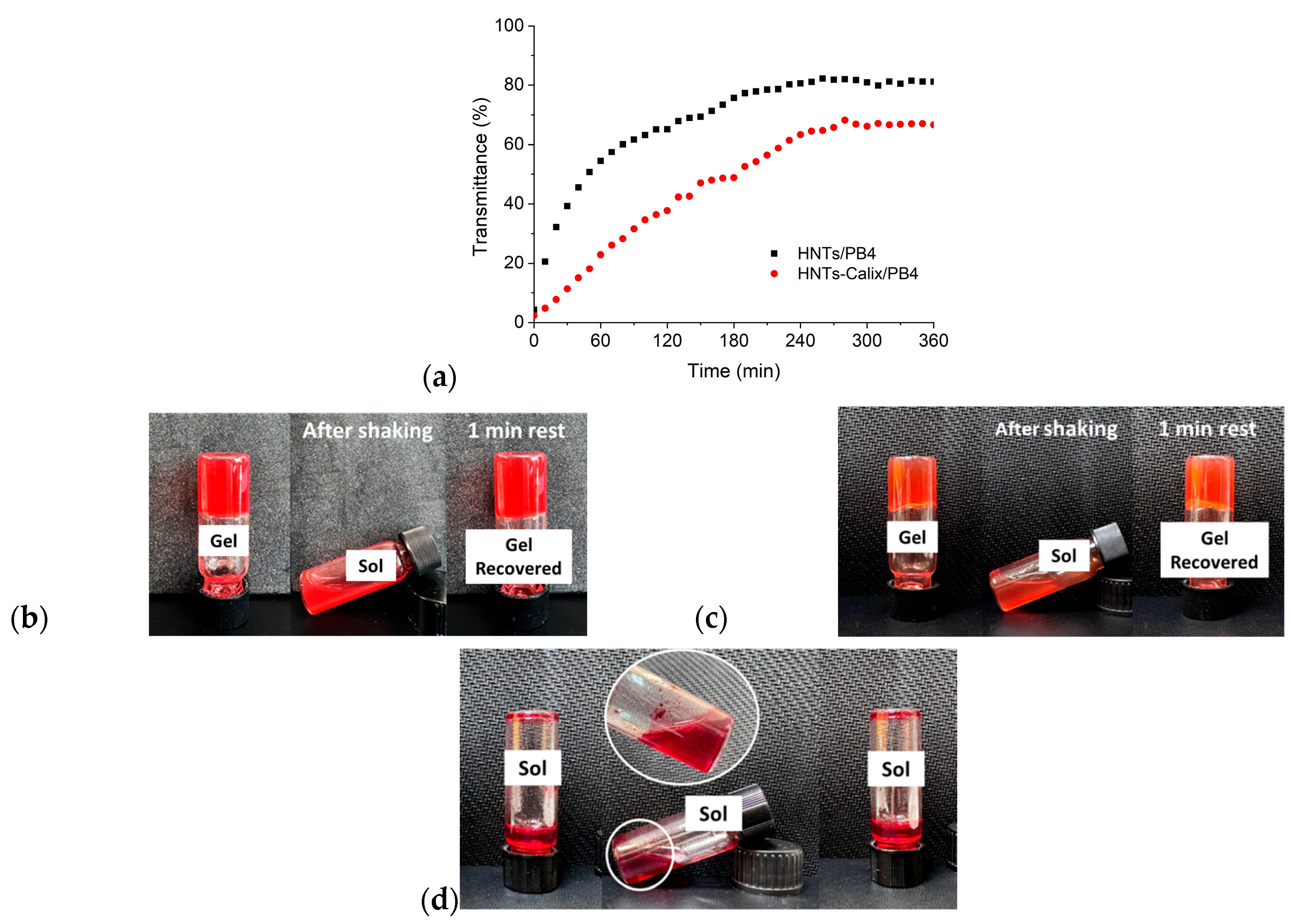
2.5. Development of Inorganic Hydrogels for Potential Biomedical Applications
3. Materials and Methods
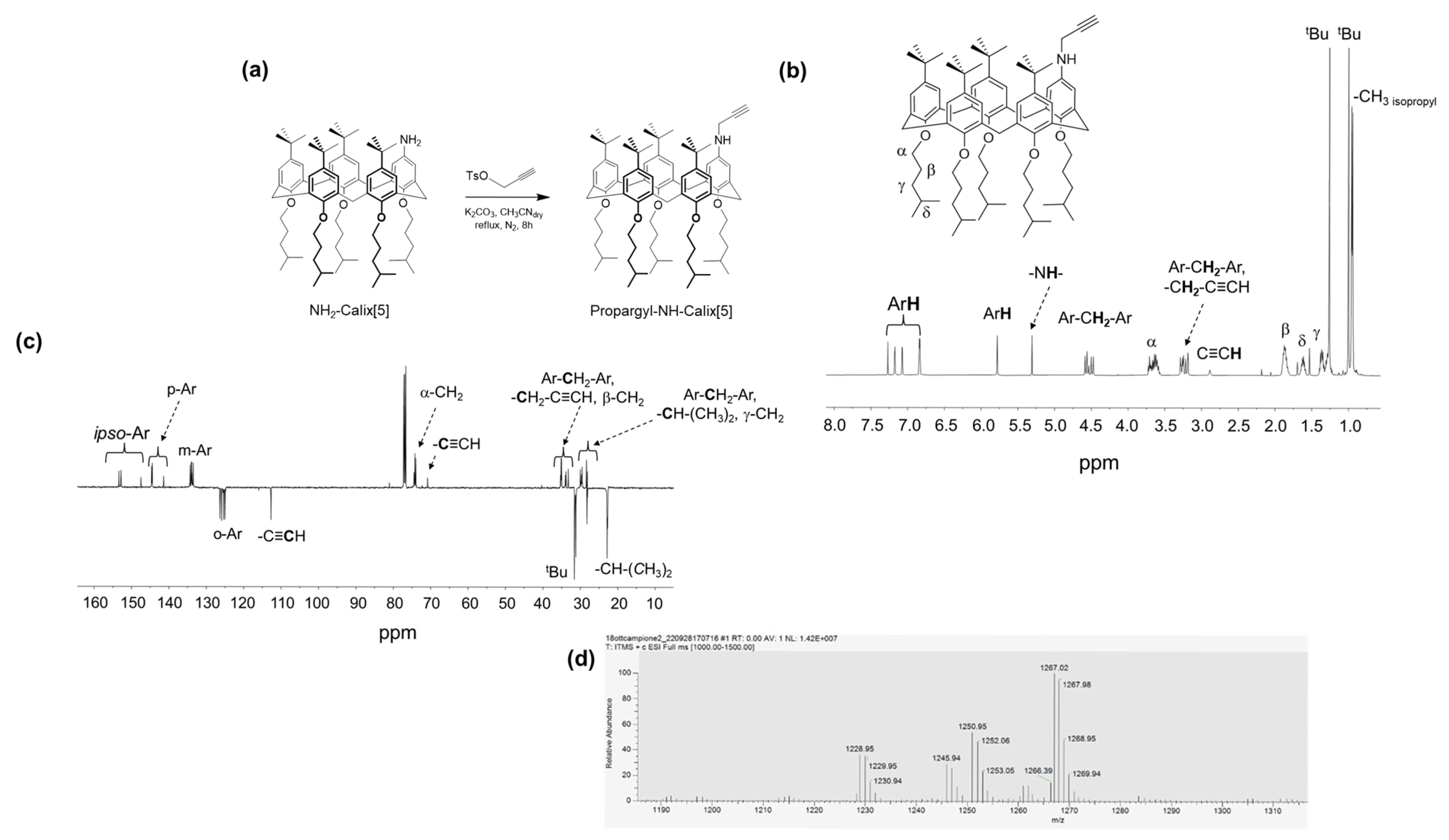
3.1. Synthesis of Propargyl-NH-Calix[5]
3.2. Synthesis of HNTs-Calix Nanomaterial
3.3. Loading of PB4 into HNTs or HNTs-Calix Nanomaterials
3.4. Kinetic Release of PB4 from HNTs/PB4 and HNTs-Calix/PB4 Nanomaterials
3.5. Adsorption Isotherm
3.6. Lap/HNTs/PB4 and Lap/HNTs-Calix/PB4 Hydrogel Preparation
3.7. Thixotropic and Sonotropic Behavior
3.8. Cell Lines
3.9. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Mangla, B.; Javed, S.; Ahsan, W.; Musyuni, P.; Sivadasan, D.; Alqahtani, S.S.; Aggarwal, G. A review of nanomaterials from synthetic and natural molecules for prospective breast cancer nanotherapy. Front. Pharmacol. 2023, 14, 1149554. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, A.; Vento, F.; Satriano, C.; Villari, V.; Micali, N.; Cucci, L.M.; Sanfilippo, V.; Mineo, P.G. Light-Triggered Polymeric Nanobombs for Targeted Cell Death. ACS Appl. Nano Mater. 2020, 3, 1950–1960. [Google Scholar] [CrossRef]
- Massaro, M.; Borrego-Sánchez, A.; Viseras-Iborra, C.; Cinà, G.; García-Villén, F.; Liotta, L.F.; Lopez Galindo, A.; Pimentel, C.; Sainz-Díaz, C.I.; Sánchez-Espejo, R.; et al. Hectorite/Phenanthroline-Based Nanomaterial as Fluorescent Sensor for Zn Ion Detection: A Theoretical and Experimental Study. Nanomaterials 2024, 14, 880. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Li, K.; Guo, B.; Gu, J.; Ta, N.; Gu, J.; Yu, H.; Sun, M.; Han, T. Emerging advances in drug delivery systems (DDSs) for optimizing cancer complications. Mater. Today Bio 2025, 30, 101375. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Neupane, Y.R.; Parvez, S.; Kohli, K. Recent Advances in Targeted Nanotherapeutic Approaches for Breast Cancer Management. Nanomedicine 2021, 16, 2605–2631. [Google Scholar] [CrossRef]
- Nicosia, A.; La Perna, G.; Cucci, L.M.; Satriano, C.; Mineo, P. A Multifunctional Conjugated Polymer Developed as an Efficient System for Differentiation of SH-SY5Y Tumour Cells. Polymers 2022, 14, 4329. [Google Scholar] [CrossRef]
- Vento, F.; Privitera, A.; Caruso, G.; Nicosia, A. A Silibinin-Poly(ε-Caprolactone) Conjugate as an Enhanced Anticancer Agent. Macromol. Biosci. 2025, 25, 2400510. [Google Scholar] [CrossRef]
- You, W.; Cai, Z.; Xiao, F.; Zhao, J.; Wang, G.; Wang, W.; Chen, Z.; Hu, W.; Chen, Y.; Wang, Z. Biomolecular Microneedle Initiates Fe3O4/MXene Heterojunction-Mediated Nanozyme-Like Reactions and Bacterial Ferroptosis to Repair Diabetic Wounds. Adv. Sci. 2025, 12, 2417314. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Akshay Kumar, K.P.; Murugesan, S.; Torres-Mendieta, R.; Wacławek, S.; Cheong, J.Y.; Černík, M.; Varma, R.S. Sustainable and safer nanoclay composites for multifaceted applications. Green Chem. 2022, 24, 3081–3114. [Google Scholar] [CrossRef]
- Peixoto, D.; Pereira, I.; Pereira-Silva, M.; Veiga, F.; Hamblin, M.R.; Lvov, Y.; Liu, M.; Paiva-Santos, A.C. Emerging role of nanoclays in cancer research, diagnosis, and therapy. Coord. Chem. Rev. 2021, 440, 213956. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Khusnetdenova, E.; Vinokurov, V.; Lvov, Y.; Fakhrullin, R. Prokaryotic and eukaryotic toxicity of halloysite decorated with photoactive nanoparticles. Chem. Commun. 2022, 58, 7719–7729. [Google Scholar] [CrossRef]
- Rozhina, E.; Panchal, A.; Akhatova, F.; Lvov, Y.; Fakhrullin, R. Cytocompatibility and cellular uptake of alkylsilane-modified hydrophobic halloysite nanotubes. Appl. Clay Sci. 2020, 185, 105371. [Google Scholar] [CrossRef]
- Cardano, F.; Massaro, M.; Leone, F.; Cinà, G.; Borbone, N.; Falanga, A.P.; Oliviero, G.; Nicosia, A.; Fresia, M.; Fin, A.; et al. Halloysite Nanotubes Functionalized with Naphthalene Diimide and Peptide Nucleic Acid Derivatives: Toward Multifunctional Nanomaterials. ACS Appl. Nano Mater. 2025, 8, 12775–12783. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; He, R.-R.; Liu, Z.; Lvov, Y.M.; Liu, M. The Horizons of Medical Mineralogy: Structure-Bioactivity Relationship and Biomedical Applications of Halloysite Nanoclay. ACS Nano 2024, 18, 20001–20026. [Google Scholar] [CrossRef]
- Caruso, M.R.; Calvino, M.M.; Cavallaro, G.; Amato, J.; Marzano, S.; D’Aria, F.; Giancola, C.; Lazzara, G.; Milioto, S.; Pagano, B. Halloysite clay nanotubes as platforms for loading of aptamers and antisense oligonucleotides. Hybrid Adv. 2025, 8, 100374. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Y.; Zhang, D.; Zhou, S.; Liu, X.; Luo, B.; Zhou, C.; Liu, M. Orally administered hydrogel containing polyphenol@halloysite clay for probiotic delivery and treatment of inflammatory bowel disease. Nano Today 2025, 62, 102669. [Google Scholar] [CrossRef]
- Drits, V.A.; Sakharov, B.A.; Hillier, S. Phase and structural features of tubular halloysite (7 Å). Clay Miner. 2018, 53, 691–720. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Wilson, I.; Keeling, J. Global occurrence, geology and characteristics of tubular halloysite deposits. Clay Miner. 2016, 51, 309–324. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Vinokurov, V.; Lvov, Y. Clay nanotube-metal core/shell catalysts for hydroprocesses. Chem. Soc. Rev. 2021, 50, 9240–9277. [Google Scholar] [CrossRef]
- Cinà, G.; Massaro, M.; Cavallaro, G.; Lazzara, G.; Sánchez-Espejo, R.; Viseras Iborra, C.; D’Abrosca, B.; Fiorentino, A.; Messina, G.M.L.; Riela, S. Development of alginate film filled with halloysite-carbon dots for active food packaging. Int. J. Biol. Macromol. 2024, 277, 134375. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, G.; Boccalon, E.; Lamberti, E.; Nocchetti, M.; Gorrasi, G. Alginate Microbeads Containing Halloysite and Layered Double Hydroxide as Efficient Carriers of Natural Antimicrobials. Nanomaterials 2024, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, S.; Liu, J.; Sun, X. Recent Advances of Halloysite Nanotubes in Biomedical Applications. Small 2024, 20, 2306169. [Google Scholar] [CrossRef]
- Fizir, M.; Dramou, P.; Dahiru, N.S.; Ruya, W.; Huang, T.; He, H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta 2018, 185, 389. [Google Scholar] [CrossRef]
- Farokh, A.; Pourmadadi, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M. Assessment of synthesized chitosan/halloysite nanocarrier modified by carbon nanotube for pH-sensitive delivery of curcumin to cancerous media. Int. J. Biol. Macromol. 2023, 237, 123937. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Cinà, G.; Cavallaro, G.; Lazzara, G.; Silvestri, A.; Barbosa, R.d.M.; Sànchez-Espejo, R.; Viseras-Iborra, C.; Notarbartolo, M.; Riela, S. Comparison of Synthetic Pathways for Obtaining Fluorescent Nanomaterials Based on Halloysite and Carbon Dots for Potential Biological Sensing. Int. J. Mol. Sci. 2024, 25, 5370. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Sabahi, H.; Hosseini, M.; Rezayan, A.H. Formulation, optimization, and characterization of naringenin-loaded halloysite nanotube to achieve enhanced antioxidant and anticancer properties. Nanomed. J. 2025, 12, 99–109. [Google Scholar] [CrossRef]
- Boraei, S.B.A.; Eshghabadi, F.; Hosseinpour, R.; Zare, Y.; Munir, M.T.; Rhee, K.Y. Halloysite nanotubes in biomedical applications: Recent approaches and future trends. Appl. Clay Sci. 2024, 253, 107346. [Google Scholar] [CrossRef]
- Massaro, M.; Ciani, R.; Grossi, G.; Cavallaro, G.; de Melo Barbosa, R.; Falesiedi, M.; Fortuna, C.G.; Carbone, A.; Schenone, S.; Sánchez-Espejo, R.; et al. Halloysite Nanotube-Based Delivery of Pyrazolo[3,4-d]pyrimidine Derivatives for Prostate and Bladder Cancer Treatment. Pharmaceutics 2024, 16, 1428. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Liu, H.; Deng, Z.; Li, J.; Ren, T.; Huang, L.; Chen, W.; Yang, Y.; Zhong, S. β-Cyclodextrin coated and folic acid conjugated magnetic halloysite nanotubes for targeting and isolating of cancer cells. Colloids Surf. B Biointerfaces 2019, 181, 379–388. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S. Organo-Clay Nanomaterials Based on Halloysite and Cyclodextrin as Carriers for Polyphenolic Compounds. J. Funct. Biomater. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Farber, M.; Rawat, V.; Diskin-Posner, Y.; Dobrovetsky, R.; Vigalok, A. Polyaromatic Calixarene Hosts: Calix[4]pyrenes. Org. Lett. 2024, 26, 5731–5735. [Google Scholar] [CrossRef]
- Lhoták, P. Upper rim-bridged calixarenes. RSC Adv. 2024, 14, 23303–23321. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, W.; Cheng, Y.; Zhou, Y.; Zou, J.; Meng, Y.; Chen, F.; Qian, Y.; Yao, Y. Recent developments in pillar[5]arene-based nanomaterials for cancer therapy. Chem. Commun. 2025, 61, 2484–2495. [Google Scholar] [CrossRef]
- Álvarez-Yebra, R.; López-Coll, R.; Clos-Garrido, N.; Lozano, D.; Lledó, A. Calix[5]arene Self-Folding Cavitands: A New Family of Bio-Inspired Receptors with Enhanced Induced Fit Behavior. Isr. J. Chem. 2024, 64, e202300077. [Google Scholar] [CrossRef]
- Carpentier, R.; Testa, C.; Pappalardo, A.; Jabin, I.; Bartik, K. Binding of Bioactive Ammonium Ions in Water with a Cavity-Based Selectivity: Water Solubilization versus Micellar Incorporation. J. Org. Chem. 2025, 90, 682–690. [Google Scholar] [CrossRef]
- Testa, C.; Gangemi, C.M.A.; Sfrazzetto, G.T.; Ricceri, M.; Giuffrida, A.; Greco, V.; Cancelliere, A.M.; Puntoriero, F.; Pappalardo, A. Luminescent Dansyl-Calix[5]arene for the Recognition of Biogenic Amines. Curr. Org. Chem. 2024, 28, 1380–1386. [Google Scholar] [CrossRef]
- Minh Hoang, C.N.; Nguyen, S.H.; Tran, M.T. Nanoparticles in cancer therapy: Strategies to penetrate and modulate the tumor microenvironment—A review. Smart Mater. Med. 2025, 6, 270–284. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Prieložná, J.; Mikušová, V.; Mikuš, P. Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles. Int. J. Pharm. X 2024, 8, 100281. [Google Scholar] [CrossRef]
- Hani, U.; Choudhary, V.T.; Ghazwani, M.; Alghazwani, Y.; Osmani, R.A.M.; Kulkarni, G.S.; Shivakumar, H.G.; Wani, S.U.D.; Paranthaman, S. Nanocarriers for Delivery of Anticancer Drugs: Current Developments, Challenges, and Perspectives. Pharmaceutics 2024, 16, 1527. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Bonaccorso, C.; Consiglio, G.; Goracci, L.; Musso, N.; Musumarra, G.; Satriano, C.; Fortuna, C.G. Modeling, design and synthesis of new heteroaryl ethylenes active against the MCF-7 breast cancer cell-line. Mol. Biosyst. 2013, 9, 2426–2429. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, D.; Musso, N.; Bonacci, P.G.; Bivona, D.A.; Massimino, M.; Stracquadanio, S.; Bonaccorso, C.; Fortuna, C.G.; Stefani, S. Heteroaryl-Ethylenes as New Lead Compounds in the Fight against High Priority Bacterial Strains. Antibiotics 2021, 10, 1034. [Google Scholar] [CrossRef]
- Brunchi, C.-E.; Morariu, S. Laponite®—From Dispersion to Gel—Structure, Properties, and Applications. Molecules 2024, 29, 2823. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Liu, Y.; Dai, J.; Wang, J.; Ju, C. Injectable laponite nanocomposite hydrogel with synergistic antibacterial and odontogenic activity for endodontic regeneration. Colloids Surf. B Biointerfaces 2025, 253, 114745. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Cinà, G.; Borrego-Sánchez, A.; Sainz-Díaz, C.I.; Viseras-Iborra, C.; Sánchez-Espejo, R.; de Melo Barbosa, R.; Leone, F.; Pibiri, I.; Noto, R.; et al. Thixotropic Hydrogels Based on Laponite® and Cucurbituril for Delivery of Lipophilic Drug Molecules. ChemPlusChem 2024, 89, e202300370. [Google Scholar] [CrossRef]
- Stealey, S.T.; Gaharwar, A.K.; Zustiak, S.P. Laponite-Based Nanocomposite Hydrogels for Drug Delivery Applications. Pharmaceuticals 2023, 16, 821. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Pchelintsev, A.; Tsygankova, L.; Tanygina, E. Freundlich Isotherm: An Adsorption Model Complete Framework. Appl. Sci. 2021, 11, 8078. [Google Scholar] [CrossRef]
- Gereli, G.; Seki, Y.; Murat Kuşoğlu, İ.; Yurdakoç, K. Equilibrium and kinetics for the sorption of promethazine hydrochloride onto K10 montmorillonite. J. Colloid Interface Sci. 2006, 299, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Duce, C.; Vecchio Ciprioti, S.; Ghezzi, L.; Ierardi, V.; Tinè, M.R. Thermal behavior study of pristine and modified halloysite nanotubes. J. Therm. Anal. Calorim. 2015, 121, 1011–1019. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes as nanoreactors for heterogeneous micellar catalysis. J. Colloid Interface Sci. 2022, 608, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Garozzo, D.; Gattuso, G.; Notti, A.; Pappalardo, A.; Pappalardo, S.; Parisi, M.F.; Perez, M.; Pisagatti, I. A Calix[5]arene-Based Heterotetratopic Host for Molecular Recognition of Long-Chain, Ion-Paired α,ω-Alkanediyldiammonium Salts. Angew. Chem. Int. Ed. 2005, 44, 4892–4896. [Google Scholar] [CrossRef]
- Görner, H.; Gruen, H. Photophysical properties of quaternary salts of 4-dialkylamino-4′-azastilbenes and their quinolinium analogues in solution: IX. J. Photochem. 1985, 28, 329–350. [Google Scholar] [CrossRef]
- Fromherz, P.; Heilemann, A. Twisted internal charge transfer in (aminophenyl)pyridinium. J. Phys. Chem. 1992, 96, 6864–6866. [Google Scholar] [CrossRef]
- Carlotti, B.; Benassi, E.; Spalletti, A.; Fortuna, C.G.; Elisei, F.; Barone, V. Photoinduced symmetry-breaking intramolecular charge transfer in a quadrupolar pyridinium derivative. Phys. Chem. Chem. Phys. 2014, 16, 13984–13994. [Google Scholar] [CrossRef]
- Benassi, E.; Carlotti, B.; Fortuna, C.G.; Barone, V.; Elisei, F.; Spalletti, A. Acid–Base Strength and Acidochromism of Some Dimethylamino–Azinium Iodides. An Integrated Experimental and Theoretical Study. J. Phys. Chem. A 2015, 119, 323–333. [Google Scholar] [CrossRef]
- Bretti, C.; Cataldo, S.; Gianguzza, A.; Lando, G.; Lazzara, G.; Pettignano, A.; Sammartano, S. Thermodynamics of Proton Binding of Halloysite Nanotubes. J. Phys. Chem. C 2016, 120, 7849–7859. [Google Scholar] [CrossRef]
- Espíndola, C.; Correa, A.J.; López-López, M.; López-Cornejo, P.; Bernal, E.; Lebrón, J.A.; Ostos, F.J.; Benhnia, M.R.-E.-I.; Moyá, M.L. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics 2022, 14, 2806. [Google Scholar] [CrossRef]
- Mancuso, A.; Massaro, M.; Leone, F.; Bonaccorsi, P.M.; Compagnini, G.; Gangemi, C.M.A.; Puntoriero, F.; Ribagorda, M.; Scardaci, V.; Viseras, C.; et al. Glucosyl OPE-Modified Halloysite Nanotubes and Their Potential as Phototherapy Agents for Bacterial Infections. Surf. Interfaces 2025, 62, 106207. [Google Scholar] [CrossRef]
- Fortuna, C.G.; Barresi, V.; Bonaccorso, C.; Consiglio, G.; Failla, S.; Trovato-Salinaro, A.; Musumarra, G. Design, synthesis and in vitro antitumour activity of new heteroaryl ethylenes. Eur. J. Med. Chem. 2012, 47, 221–227. [Google Scholar] [CrossRef] [PubMed]

| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Qm (10−5 mol g−1) | KL (105 L mol−1) | R2 | KF (10−4 mol g−1 (mol L−1)1/n) | n | R2 |
| 7 ± 1 | 1.4 ± 0.9 | 0.8623 | 9 ± 3 | 3.7 ± 0.4 | 0.9825 |
| Nanomaterial | pH | DEM | First Order | Power Fit | |||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | k2 (min−1) | R2 | k1 (min−1) | R2 | kF (min−1) | n | R2 | ||
| HNTs/PB4 | 1.0 | n.a. | n.a. | / | 0.026 ± 0.002 | 0.9806 | 12 ± 1 | 0.42 ± 0.03 | 0.9746 |
| 7.0 | n.a. | n.a. | / | 0.0038 ± 0.0005 | 0.9473 | 0.26 ± 0.03 | 0.43 ± 0.02 | 0.9799 | |
| HNTs-Calix/PB4 | 1.0 | 0.038 ± 0.001 | 0.0017 ± 0.0006 | 0.9974 | 0.029 ± 0.002 | 0.9796 | 8 ± 1 | 0.02 ± 0.03 | 0.8765 |
| 7.0 | 0.06 ± 0.01 | 0.0017 ± 0.0004 | 0.9888 | 0.013 ± 0.003 | 0.8792 | 0.38 ± 0.03 | 0.30 ± 0.01 | 0.9894 | |
| Nanomaterials | IC50 (µM) |
|---|---|
| Lap/HNTs/PB4 | 10.0 |
| Lap/HNTs-Calix/PB4 | 21.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinà, G.; Massaro, M.; Pappalardo, A.; Bonaccorso, C.; Fortuna, C.G.; Mineo, P.G.; Nicosia, A.; Poma, P.; Sánchez-Espejo, R.; Testa, C.; et al. Covalently Functionalized Halloysite-Calixarene Nanotubes for Injectable Hydrogels: A Multicavity Platform for Hydrophobic Drug Delivery. Pharmaceuticals 2025, 18, 1356. https://doi.org/10.3390/ph18091356
Cinà G, Massaro M, Pappalardo A, Bonaccorso C, Fortuna CG, Mineo PG, Nicosia A, Poma P, Sánchez-Espejo R, Testa C, et al. Covalently Functionalized Halloysite-Calixarene Nanotubes for Injectable Hydrogels: A Multicavity Platform for Hydrophobic Drug Delivery. Pharmaceuticals. 2025; 18(9):1356. https://doi.org/10.3390/ph18091356
Chicago/Turabian StyleCinà, Giuseppe, Marina Massaro, Andrea Pappalardo, Carmela Bonaccorso, Cosimo G. Fortuna, Placido G. Mineo, Angelo Nicosia, Paola Poma, Rita Sánchez-Espejo, Caterina Testa, and et al. 2025. "Covalently Functionalized Halloysite-Calixarene Nanotubes for Injectable Hydrogels: A Multicavity Platform for Hydrophobic Drug Delivery" Pharmaceuticals 18, no. 9: 1356. https://doi.org/10.3390/ph18091356
APA StyleCinà, G., Massaro, M., Pappalardo, A., Bonaccorso, C., Fortuna, C. G., Mineo, P. G., Nicosia, A., Poma, P., Sánchez-Espejo, R., Testa, C., Viseras, C., & Riela, S. (2025). Covalently Functionalized Halloysite-Calixarene Nanotubes for Injectable Hydrogels: A Multicavity Platform for Hydrophobic Drug Delivery. Pharmaceuticals, 18(9), 1356. https://doi.org/10.3390/ph18091356














