Effects of CCL20/CCR6 Modulators in a T Cell Adoptive Transfer Model of Colitis
Abstract
1. Introduction
2. Results
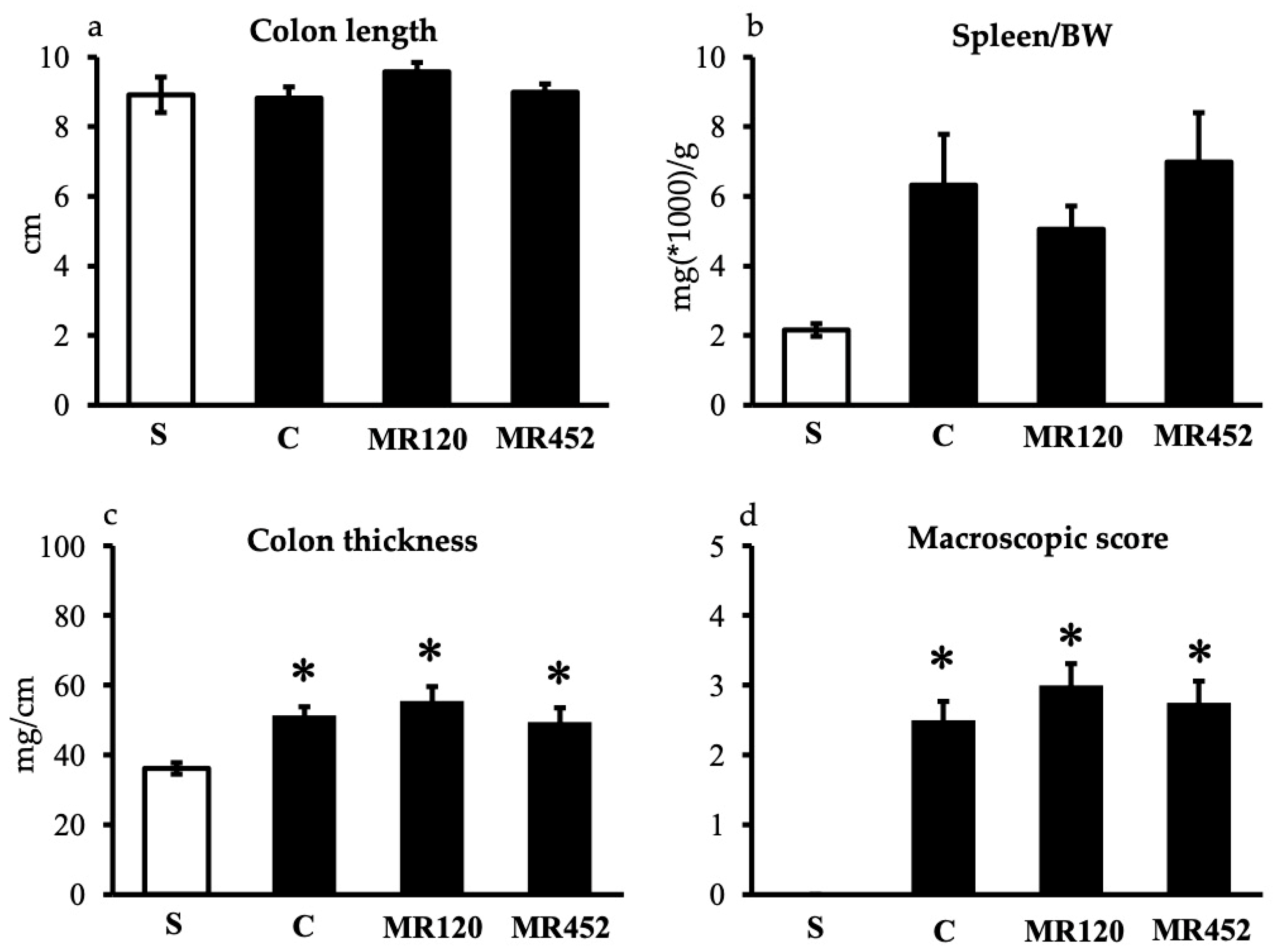
2.1. Effects on Inflammatory Responses
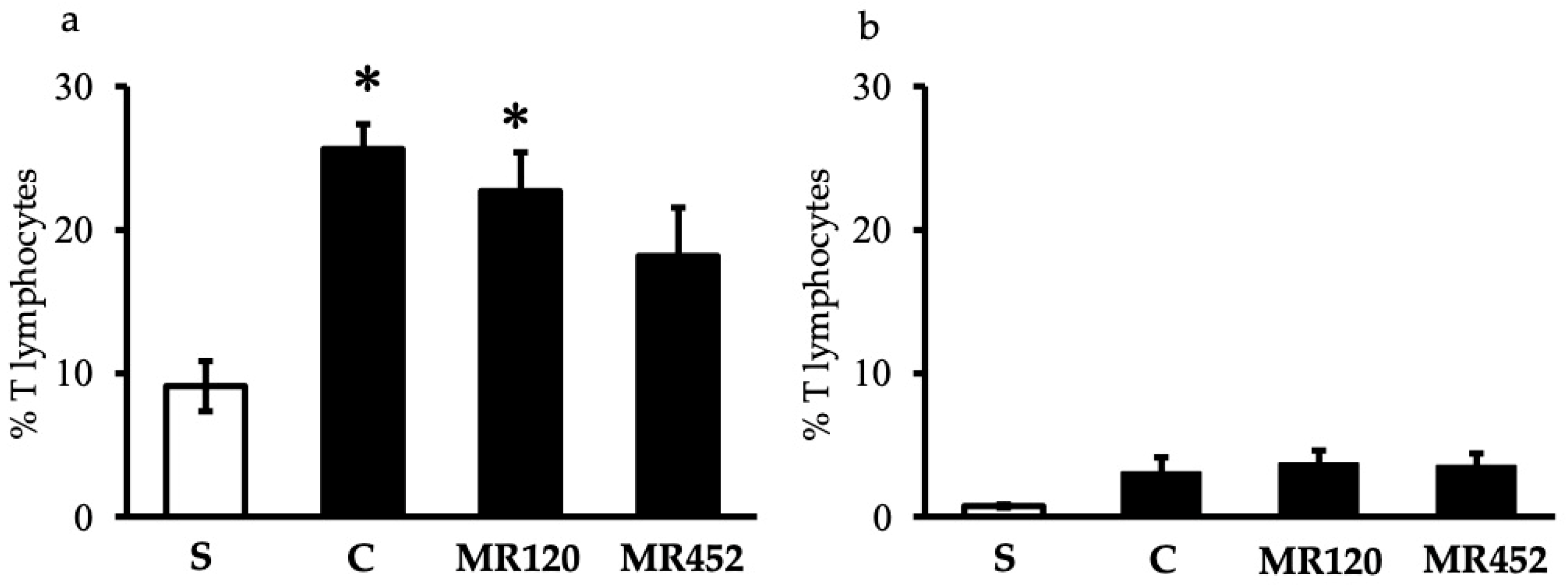
2.2. Effects on T Lymphocytes
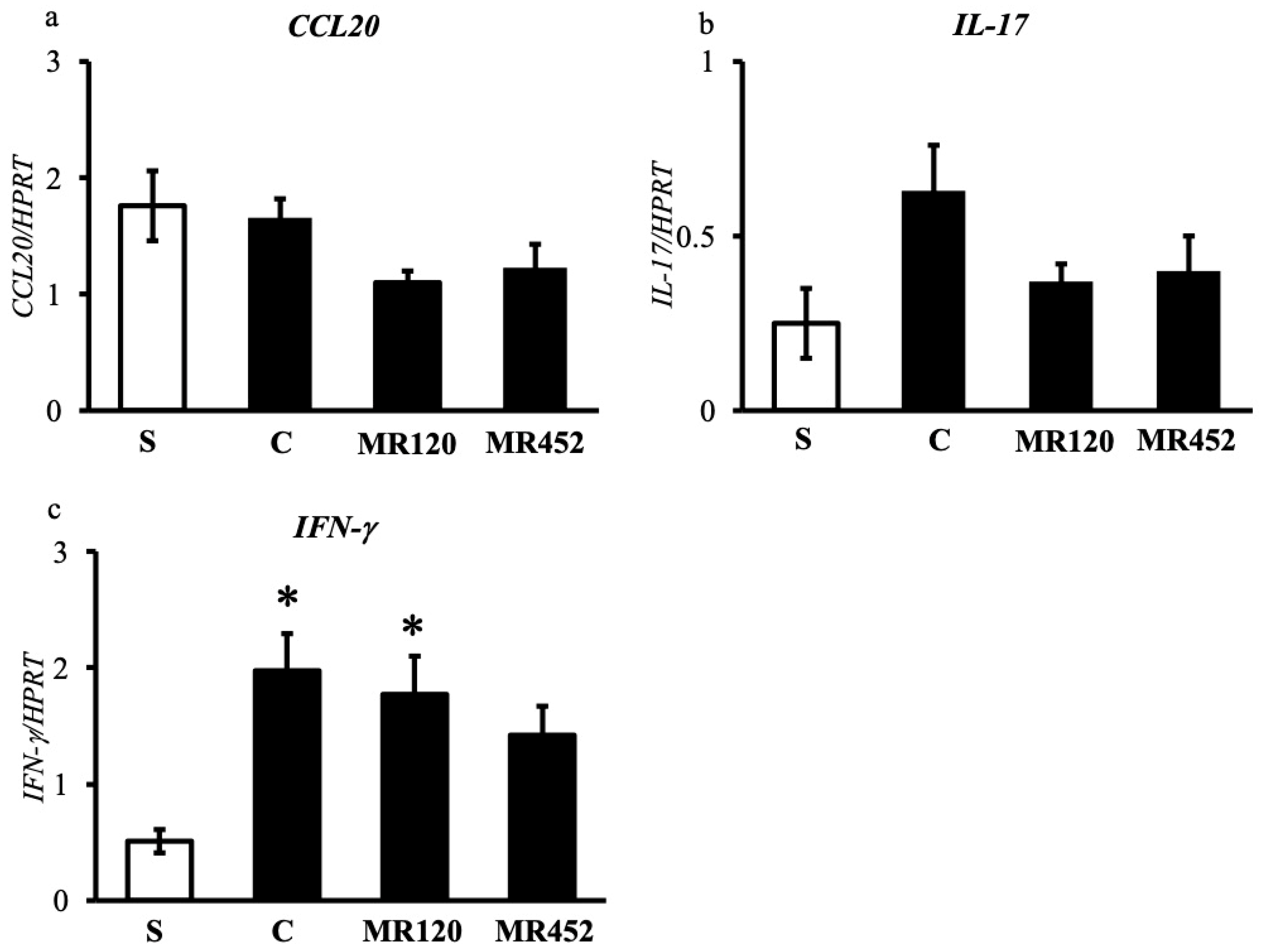
2.3. Effects on Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Development of Colitis
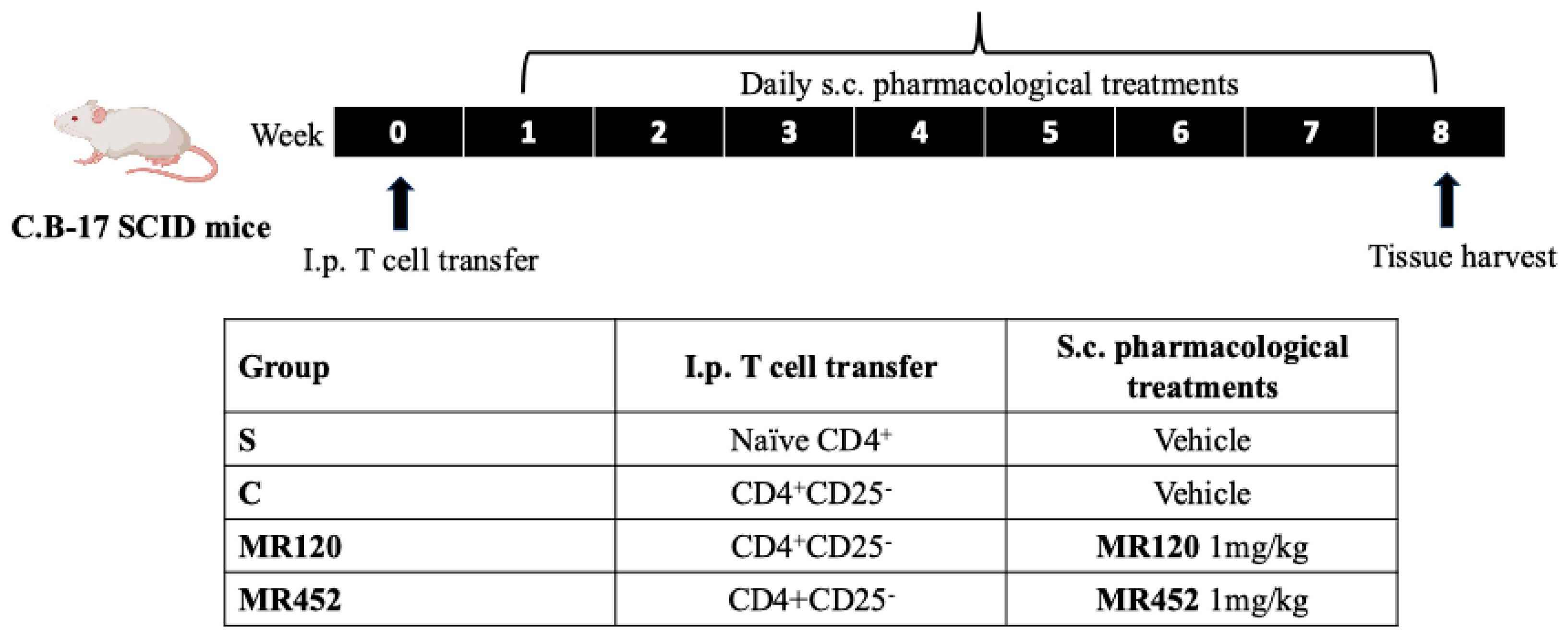
4.3. Experimental Design
4.4. Evaluation of Inflammatory Responses
4.4.1. Disease Activity Index (DAI)
- ○
- Stool consistency: 0 (normal), 1 (soft), and 2 (liquid);
- ○
- Rectal bleeding: 0 (no bleeding), 1 (light bleeding), and 2 (heavy bleeding);
- ○
- Body weight loss: 0 (<5%), 1 (5–10%), 2 (10–15%), 3 (15–20%), 4 (20–25%), and 5 (>25%).
4.4.2. Colon Length and Thickness
4.4.3. Macroscopic Score
4.4.4. Spleen/Body Weight Ratio
4.4.5. Colon and Lung MPO Activity
4.4.6. Flow Cytometry Assays
Isolation of Splenocytes
Isolation of Mesenteric Lymph Nodes (MLNs)
Immunofluorescent Staining
4.4.7. Analysis of Inflammatory Cytokines
ELISA Assays
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
- ○
- HPRT:
- ○
- CCL20:
- ○
- IL-17:
- ○
- INF-γ:
4.5. Statistics
4.6. Materials
- CD4+CD25+ regulatory T cell isolation kit-cod. 130-091-041 (MACS Milteny Biotec, Bergisch Gladbach, Germany).
- Halt Protease Inhibitor Cocktail 100x-cod. 78429 (ThermoFisher Scientific, Waltham, MA, USA).
- Hexadecylthrimethyl-ammonium bromide (HTAB) cod. H5882 (Sigma-Aldrich™, St. Louis, MO, USA).
- Phycoerythrin-cyanine 5 (PE-Cy5) conjugated anti-mouse CD3ε (catalog number 15-0031, lot number B226301); fluorescein isothiocyanate (FITC) anti-mouse CD4 (catalog number 100406, lot number B210488); propidium iodide were purchased from BioLegend™ (San Diego, CA, USA).
- IgG1-Fc was purchased from Millipore™ (Merck, Darmstadt, Germany).
- Hank’s Balanced Salt Solution (HBSS) and fetal calf serum (FCS) were purchased from Euroclone (Milano, Italy).
- Mouse MIP-3a ELISA Kit, cod. EM1210 (Wuhan Fine Biotech Co., Ltd.; Wuhan, Cina).
- Mouse IL-6 ELISA Kit, cod. 126ELM-IL6-CL-1 (RayBiotech, Peachtree Corners, GA, USA).
- Mouse Interleukin 1β ELISA Kit, cod. E08054 (Cusabio, Houston, TX, USA).
- Bicinchoninic acid (BCA) protein assay kit, cod. 23225 (ThermoFisher Scientific Inc., Waltham, MA, USA).
- Qiagen RNeasy Protect Mini Kit, cod. 74104 (Qiagen, Hilden, Germany).
- OneStep RT-PCR kit, cod. 210212 (Qiagen, Hilden, Germany).
- Primers were purchased from Life Technologies, ThermoFisher Scientific Inc., Waltham, MA, USA.
- Hydrogen peroxide, tetramethylbenzidine, dimethyl formamide, EDTA, and all the other reagents were purchased from Sigma-Aldrich™, St. Louis, MO, USA.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBDs | inflammatory bowel diseases |
| CCL20 | CC chemokine ligand 20 |
| CCR6 | C-C chemokine receptor type 6 |
| TNBS | 2,4,6-trinitrobenzene sulfonic acid |
| DAI | disease activity index |
| DSS | dextran sulfate sodium |
| CD | Crohn’s disease |
| S | sham mice |
| C | colitic mice |
| BW | body weight |
| MPO | myeloperoxidase |
| i.p. | intraperitoneal |
| MLN | mesenteric lymph nodes |
| SPF | specific pathogen free |
| PBS | phosphate-buffered saline |
| s.c. | subcutaneous |
| DMSO | dimethyl sulfoxide |
| RCF | relative centrifugal force |
| HTAB | hexadecylthrimethyl-ammonium bromide |
| FCS | fetal calf serum |
| FITC | fluorescein isothiocyanate |
| PI | propidium iodide |
| FSC | forward scatter |
| SSC | side scatter |
| ELISA | enzyme-linked immunosorbent assay |
| BCA | bicinchoninic acid |
| RT-PCR | reverse transcription polymerase chain reaction |
| SEM | standard error of the mean |
References
- Hracs, L.; Windsor, C.D.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinović, A.; et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Bolinger, A.A.; Chen, H.; Liu, Z.; Cong, Y.; Brasier, A.R.; Pinchuk, I.V.; Tian, B.; Zhou, J. Target-based small molecule drug discovery towards novel therapeutics for inflammatory bowel diseases. Inflamm. Bowel Dis. 2021, 27, S38–S62. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, T.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Eastaff-Leung, N.; Mabarrack, N.; Barbour, A.; Cummins, A.; Barry, S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 2010, 30, 80–89. [Google Scholar] [CrossRef]
- Lee, A.Y.S.; Eri, R.; Lyons, A.B.; Grimm, M.C.; Korner, H. CC chemokine ligand 20 and its cognate receptor CCR6 in mucosal T cell immunology and inflammatory bowel disease: Odd couple or axis of evil? Front. Immunol. 2013, 4, 194. [Google Scholar] [CrossRef]
- Skovdahl, H.K.; Granlund, A.B.; Ostvik, A.E.; Bruland, T.; Bakke, I.; Torp, S.H.; Damås, J.K.; Sandvik, A.K. Expression of CCL20 and its corresponding receptor CCR6 is enhanced in active Inflammatory Bowel Disease, and TLR3 mediates CCL20 expression in colonic epithelial cells. PLoS ONE 2015, 10, e0141710. [Google Scholar] [CrossRef]
- Skovdahl, H.K.; Dams, J.K.; van Beelen Granlund, A.; Ostvik, A.E.; Doseth, B.; Bruland, T.; Mollnes, T.E.; Sandvik, A.K. C-C motif ligand 20 (CCL20) and C-C motif chemokine receptor 6 (CCR6) in human peripheral blood mononuclear cells: Dysregulated in ulcerative colitis and a potential role for CCL20 in IL-1β release. Int. J. Mol. Sci. 2018, 19, 3257. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Katchar, K.; Kelly, C.P.; Keates, S.; O’Brien, M.J.; Keates, A.C. MIP-3alpha neutralizing monoclonal antibody protects against TNBS-induced colonic injury and inflammation in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1263–G1271. [Google Scholar] [CrossRef]
- Varona, R.; Cadenas, V.; Flores, J.; Martinez, A.C.; Marquez, G. CCR6 has a non-redundant role in the development of inflammatory bowel disease. Eur. J. Immunol. 2003, 33, 2937–2946. [Google Scholar] [CrossRef]
- Kulkarni, N.; Meitei, H.T.; Sonar, S.A.; Sharma, P.K.; Mujeeb, V.R.; Srivastava, S.; Boppana, R.; Lal, G. CCR6 signaling inhibits suppressor function of induced-Treg during gut inflammation. J. Autoimmun. 2018, 88, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.G.; Giorgio, C.; Allodi, M.; Palese, S.; Barocelli, E.; Ballabeni, V.; Szpakowska, M.; Chevigné, A.; van Hamburg, J.P.; Davelaar, N.; et al. Discovery of small-molecules targeting the CCL20/CCR6 axis as first-in-class inhibitors for inflammatory bowel diseases. Eur. J. Med. Chem. 2022, 243, 114703. [Google Scholar] [CrossRef]
- Allodi, M.; Giorgio, C.; Incerti, M.; Corradi, D.; Flammini, L.; Ballabeni, V.; Barocelli, E.; Radi, M.; Bertoni, S. Probing the effects of MR120 in preclinical chronic colitis: A first-in-class anti-IBD agent targeting the CCL20/CCR6 axis. Eur. J. Pharmacol. 2023, 945, 175613. [Google Scholar] [CrossRef]
- Yoo, K.D.; Yu, M.Y.; Kim, K.H.; Lee, S.; Park, E.; Kang, S.; Lim, D.-H.; Lee, Y.; Song, J.; Kown, S.; et al. Role of the CCL20/CCR6 axis in tubular epithelial cell injury: Kidney-specific translational insights from acute kidney injury to chronic kidney disease. FASEB J. 2024, 38, e23407. [Google Scholar] [CrossRef]
- Tawaraishi, T.; Sakauchi, N.; Hidaka, K.; Yoshikawa, K.; Okui, T.; Kuno, H.; Chisaki, I.; Aso, K. Identification of a novel series of potent and selective CCR6 inhibitors as biological probes. Bioorg. Med. Chem. Lett. 2018, 28, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Martina, M.G.; Giorgio, C.; Chiara, M.L.; Allodi, M.; Durante, J.; Bertoni, S.; Radi, M. Benzofuran-2-carboxamide derivatives as immunomodulatory agents blocking the CCL20-infuced chemotaxis and colon cancer growth. ChemMedChem 2024, 19, e202400389. [Google Scholar] [CrossRef]
- Powrie, F.; Leach, M.W.; Mauze, S.; Caddle, L.B.; Coffman, R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993, 5, 1461–1471. [Google Scholar] [CrossRef]
- te Velde, A.A.; de Kort, F.; Sterrenburg, E.; Pronk, I.; ten Kate, F.J.W.; Hommes, D.W.; van Deventer, S.J.H. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Kjellev, S.; Lundsgaard, D.; Poulsen, S.S.; Markholst, H. Reconstitution of scid mice with CD4+CD25− T cells leads to rapid colitis: An improved model for pharmacologic testing. Int. Immunopharmacol. 2006, 6, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Valatas, V.; Bamias, G.; Kolios, G. Experimental colitis models: Insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur. J. Pharmacol. 2015, 759, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Fuss, I.; Kelsall, B.L.; Stüber, E.; Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995, 182, 1281–1290. [Google Scholar] [CrossRef]
- Hughes, C.C.W.; Mestas, J. Chapter 15—The mouse trap: How well do mice model human immunology? In The Mouse in Biomedical Research, 2nd ed.; American College of Laboratory Animal Medicine; Academic Press: Cambridge, MA, USA, 2007; Volume IV, pp. 303–312. [Google Scholar] [CrossRef]
- Read, S.; Malström, V.; Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000, 192, 295–302. [Google Scholar] [CrossRef]
- Kitamura, K.; Farber, J.M.; Kelsall, B.L. CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J. Immunol. 2010, 185, 3295–3304. [Google Scholar] [CrossRef]
- Ostanin, D.V.; Bao, J.; Koboziev, I.; Gray, L.; Robinson-Jackson, S.A.; Kosloski-Davidson, M.; Price, V.H.; Grisham, M.B. T cell transfer model of chronic colitis: Concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G135–G416. [Google Scholar] [CrossRef]
- Knight, Z.A.; Shokat, K.M. Chemical genetics: Where genetics and pharmacology meet. Cell 2007, 128, 425–430. [Google Scholar] [CrossRef]
- Lindebo Holm, T.; Poulsen, S.S.; Markholst, H.; Reedtz-Runge, S. Pharmacological evaluation of the SCID T cell transfer model of colitis: As a model of Crohn’s disease. Int. J. Inflamm. 2012, 2012, 412178. [Google Scholar] [CrossRef]
- Scott, F.L.; Clemons, B.; Brooks, J.; Brahmachary, E.; Powell, R.; Dedman, H.; Desale, H.G.; Timony, G.A.; Martinborough, E.; Rosen, H.; et al. Ozanimod (RCP1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br. J. Pharmacol. 2016, 173, 1778–1792. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Bischoff, S.C.; Mailer, R.; Pabst, O.; Weier, G.; Sedlik, W.; Li, Z.; Chen, J.J.; Murphy, D.L.; Gershon, M.D. Role of serotonin in intestinal inflammation: Knock out of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G685–G695. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, C.; Allodi, M.; Palese, S.; Grandi, A.; Tognolini, M.; Castelli, R.; Lodola, A.; Flammini, L.; Cantoni, A.M.; Barocelli, E.; et al. UniPR1331: Small Eph/Ephrin Antagonist Beneficial in Intestinal Inflammation by Interfering with Type-B Signaling. Pharmaceuticals 2021, 14, 502. [Google Scholar] [CrossRef] [PubMed]
- Ivey, C.L.; Williams, F.M.; Collins, P.D.; Jose, P.J.; Williams, T.J. Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. J. Clin. Investig. 1995, 95, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Kruisbeek, A.M. Isolation of mouse mononuclear cells. Curr. Protoc. Immunol. 2001, 39, 3.1.1–3.1.5. [Google Scholar] [CrossRef]




| S | C | MR120 | MR452 | |
|---|---|---|---|---|
| %CD4+ T lymphocytes | 80.5 ± 3.4 | 87.4 ± 2.1 | 87.5 ± 2.8 | 77.5 ± 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allodi, M.; Flammini, L.; Giorgio, C.; Martina, M.G.; Barbieri, F.; Ballabeni, V.; Barocelli, E.; Radi, M.; Bertoni, S. Effects of CCL20/CCR6 Modulators in a T Cell Adoptive Transfer Model of Colitis. Pharmaceuticals 2025, 18, 1327. https://doi.org/10.3390/ph18091327
Allodi M, Flammini L, Giorgio C, Martina MG, Barbieri F, Ballabeni V, Barocelli E, Radi M, Bertoni S. Effects of CCL20/CCR6 Modulators in a T Cell Adoptive Transfer Model of Colitis. Pharmaceuticals. 2025; 18(9):1327. https://doi.org/10.3390/ph18091327
Chicago/Turabian StyleAllodi, Marika, Lisa Flammini, Carmine Giorgio, Maria Grazia Martina, Francesca Barbieri, Vigilio Ballabeni, Elisabetta Barocelli, Marco Radi, and Simona Bertoni. 2025. "Effects of CCL20/CCR6 Modulators in a T Cell Adoptive Transfer Model of Colitis" Pharmaceuticals 18, no. 9: 1327. https://doi.org/10.3390/ph18091327
APA StyleAllodi, M., Flammini, L., Giorgio, C., Martina, M. G., Barbieri, F., Ballabeni, V., Barocelli, E., Radi, M., & Bertoni, S. (2025). Effects of CCL20/CCR6 Modulators in a T Cell Adoptive Transfer Model of Colitis. Pharmaceuticals, 18(9), 1327. https://doi.org/10.3390/ph18091327







