Antidiabetic Properties of the Tropical Tree Schinus molle L. (pirul): A Comprehensive Review
Abstract
1. Introduction
2. Overview of S. molle
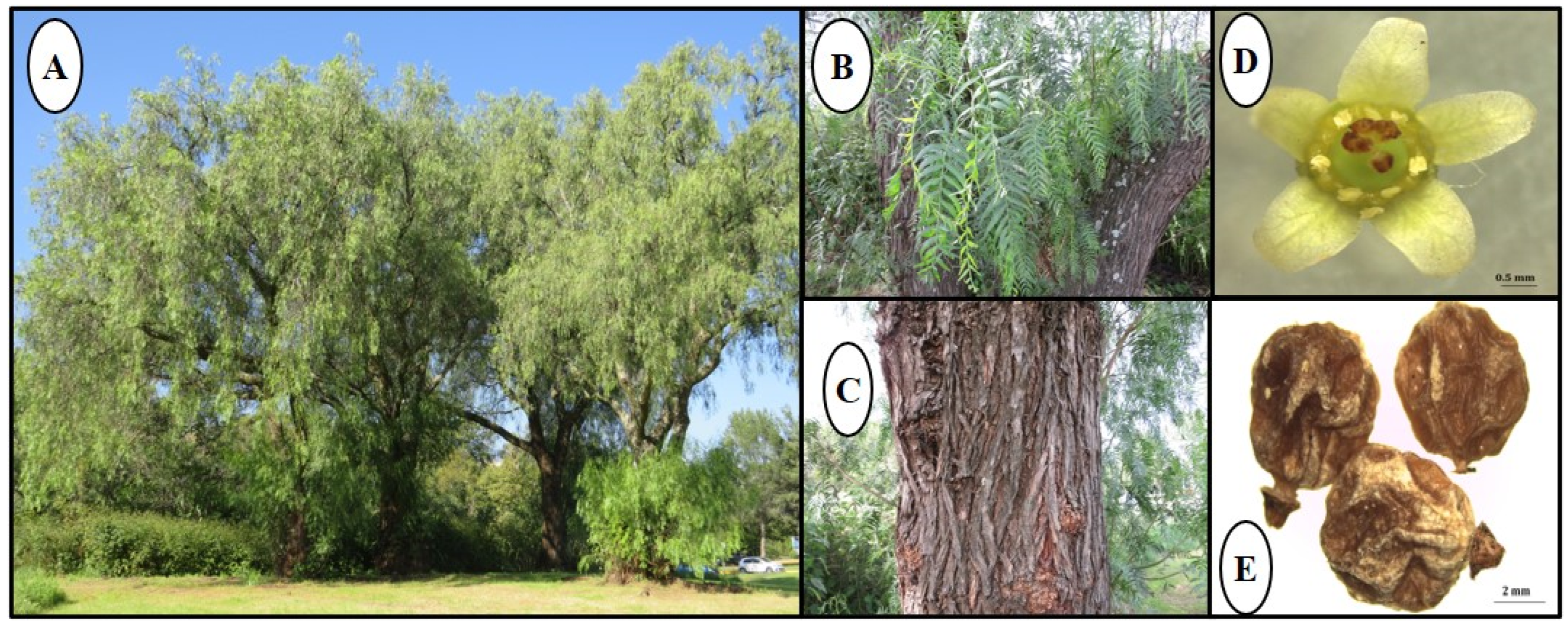
2.1. Botanical Description and Geographical Distribution
2.2. Uses and Biological Properties of Pirul
3. Therapeutic Properties of Pirul as Related to DM
3.1. Inhibition of the Enzymes α-Glucosidase and α-Amylase
3.2. Anti-Inflammatory Effect
3.3. Antioxidant Effect
| Part of the Plant/ Extract or Fraction | Compound | Concentration | Country of the Sample | Reference |
|---|---|---|---|---|
| Fruits/Methanolic extract | Masazino-flavanone | 1177.65 μg/g | Tunisia | [40] |
| Fruits/Monosaccharide fraction | Arabinose | 40.55% | [41] | |
| Galacturonic acid | 41.15% | Tunisia | ||
| Fucose | 10.90% | |||
| Galactose | 7.40% | |||
| Fruits/Aqueous extract | Chlorogenic acid | 0.19 ± 0.01 (mg/gdw) | [42] | |
| Ellagic acid | 0.124 ± 0.002 (mg/gdw) | Peru | ||
| Quercetin derivatives | 0.42 ± 0.06 (mg/gdw) | |||
| Fruits/Methanolic extract | 11507.21 ± 90.5 (mg/100 g) | Brazil | [55] | |
| Fructose | 9528.74 ± 46.67 (mg/100 g) | India | ||
| 11829.82 ± 23.73 (mg/100 g) | Sri Lanka | |||
| 9816.07 ± 36.51 (mg/100 g) | Brazil | |||
| Glucose | 6181.37 ± 315.61 (mg/100 g) | India | ||
| 9758.15 ± 330.28 (mg/100 g) | Sri Lanka | |||
| 134.60 ± 3.20 (mg/100 g) | Brazil | |||
| Piperine | 101.10 ± 2.84 (mg/100 g) | India | ||
| 120.67 ± 1.91 (mg/100 g) | Sri Lanka | |||
| 526.72 ± 6.06 (mg/100 g) | Brazil | |||
| Gallic Acid | 657.59 ± 5.25 (mg/100 g) | India | ||
| 168.15 ± 1.43 (mg/100 g) | Sri Lanka | |||
| 144.85 ± 0.71 (mg/100 g) | Brazil | |||
| Protocatechuic Acid | 237.52 ± 0.64 (mg/100 g) | India | ||
| 29.47 ± 0.18 (mg/100 g) | Sri Lanka | |||
| 85.91 ± 2.88 (mg/100 g) | Brazil | |||
| Epicatechin | 89.24 ± 2.04 (mg/100 g) | India | ||
| 38.26 ± 1.28 (mg/100 g) | Sri Lanka | |||
| 115.92 ± 5.00(mg/100 g) | Brazil | |||
| p-Coumaric Acid | 151.33 ± 7.07(mg/100 g) | India | ||
| 48.24 ± 1.28(mg/100 g) | Sri Lanka | |||
| Leaves/Essential oils | α-Phellandrene | 25.9% | Portugal | [56] |
| Limonene | 11.7% | |||
| Myrcene | 11.1% | |||
| β-Phellandrene | 10.5% | |||
| Elemol | 9.0% | |||
| Fruits/Essential oils | β-myrcene | 51.3% | Portugal | [56] |
| Limonene | 14.1% | |||
| α-Phellandrene | 14.0% | |||
| β-Phellandrene | 11.0% | |||
| Wood branches/Essential oils | α-Elemol | 14.79% | Egypt | [57] |
| β-Pinene | 13.39% | |||
| Myrcene | 12.26% | |||
| α-Phellandrene | 10.41% | |||
| Caryophyllene | 7.69% | |||
| Seeds/Dichloromethane extract and fractions | Isomasticadienonic acid | n/i | South Africa | [49] |
| Masticatrienonate | n/i | |||
| Fruits/Triterpens and biflavanone | 3-epi-Isomasticadienolalic acid | n/i | Spain | [51] |
| Isomasticadienonalic acid | n/i | |||
| Chamaejasmin | n/i | |||
| Seeds/Methanolic extract | Bis (2-ethylhexyl) phthalate | 59.11% | Saudi Arabia | [58] |
| n-Hexadecanoic acid | 10.84% | |||
| Leaves/Methanolic extract | Squalene | 16.87% | Saudi Arabia | [58] |
| Azulene | 14.88% | |||
| Lupeol | 12.4% |
3.4. Toxicity of Pirul
4. Discussion
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 10 October 2025).
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef]
- Serina, J.J.C.; Castilho, P.C.M.F. Using polyphenols as a relevant therapy to diabetes and its complications, a review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8355–8387. [Google Scholar] [CrossRef]
- Kaur, R.; Sood, A.; Lang, D.K.; Arora, R.; Kumar, N.; Diwan, V.; Saini, B. Natural Products as Sources of Multitarget Compounds: Advances in the Development of Ferulic Acid as Multitarget Therapeutic. Curr. Top. Med. Chem. 2022, 22, 347–365. [Google Scholar] [CrossRef]
- Escandón-Rivera, S.M.; Mata, R.; Andrade-Cetto, A. Molecules Isolated from Mexican Hypoglycemic Plants: A Review. Molecules 2020, 25, 4145. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose Response 2019, 17, 1559325819852243. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, R.; Cáceres, A.; Cruz, S.M.; Aceituno, J.A.; Marroquín, E.S.; Barrios Sosa, A.C.; Strangman, W.K.; Williamson, R.T. Nephroprotective plant species used in traditional Mayan Medicine for renal-associated diseases. J. Ethnopharmacol. 2023, 301, 115755. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Glenn, A. Traditional knowledge for modern ailments–plants used for the treatment of diabetes and cancer in Northern Peru. J. Med. Plant Res. 2011, 5, 6916–6930. [Google Scholar]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:71044-1/general-information (accessed on 10 October 2025).
- Atlas de las Plantas de la Medicina Tradicional Mexicana. Biblioteca Digital de la Medicina Tradicional Mexicana, Universidad Nacional Autónoma de México. Pirul. Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=pirul (accessed on 10 October 2025).
- Machado, C.D.; Ramanb, V.; Junaid, U.; Rehmanb, J.U.; Maiac, B.H.L.N.S.; Meneghettic, E.K.; Almeidaa, V.P.; Silva, R.Z.; Faragoa, P.V.; Khanb, I.A.; et al. Schinus molle: Anatomy of leaves and stems, chemical composition and insecticidal activities of volatile oil against bed bug (Cimex lectularius). Rev. Bras. Farmacogn. 2018, 29, 1–10. [Google Scholar] [CrossRef]
- Barkley, F.A. A study of Schinus L. Brittonia 1957, 5, 160–198. [Google Scholar] [CrossRef]
- World Flora Online. Available online: https://www.worldfloraonline.org/taxon/wfo-0000435157 (accessed on 10 October 2025).
- Mompha, L.M.M.; Palacios, C.R.R.; de Nova Vázquez, J.A.; Alvarado, F.; Mireles, L.E.M.; Gallardo, R.B. Relationship between tree cover and tree diversity in urban parks in a semiarid city in Mexico. ARPHA Prepr. 2025, 6, e167706. [Google Scholar]
- Goldstein, D.J.; Coleman, R.C. Schinus molle L. (Anacardiaceae) Chicha production in the Central Andes. Econ. Bot. 2004, 58, 523–529. [Google Scholar] [CrossRef]
- Rodríguez Carrasco, J.; García-Godos, A.P. Capacidad probiótica de bacterias lácticas aisladas de chicha de molle. Rev. Soc. Quím. Perú 2017, 83, 391–402. [Google Scholar] [CrossRef]
- Kramer, F.L. The pepper tree, Schinus molle L. Econ. Bot. 1957, 11, 322–326. [Google Scholar]
- Antúnez de Mayolo, K.K. Peruvian natural dye plants. Econ. Bot. 1989, 43, 181–191. [Google Scholar] [CrossRef]
- Ramírez-Albores, J.E.; Richardson, D.M.M.; Stefenon, V.M.; Bizama, G.A.; Pérez-Suárez, M.; Badano, E.I. A global assessment of the potential distribution of naturalized and planted populations of the ornamental alien tree Schinus molle. NeoBiota 2021, 68, 105–126. [Google Scholar] [CrossRef]
- Murray, P.; Murray, G. Phytochemistry, traditional uses and bioactivity of the medicinal plant Schinus areira L. (Anacardiaceae): A review. Nat. Prod. J. 2017, 7, 97–103. [Google Scholar] [CrossRef]
- Villavicencio-Nieto, M.A.; Pérez-Escandón, B.E.; Gordillo-Martínez, A.J. Plantas tradicionalmente usadas como plaguicidas en el estado de Hidalgo, México. Polibotánica 2010, 30, 193–238. [Google Scholar]
- Huerta, A.; Chiffelle, I.; Puga, K.; Azúa, F.; Araya, J.E. Toxicity and repellence of aqueous and ethanolic extracts from Schinus molle on elm leaf beetle Xanthogaleruca luteola. Crop Prot. 2010, 29, 1118–1123. [Google Scholar] [CrossRef]
- Islam, J.; Zaman, K.; Duarah, S.; Raju, P.S.; Chattopadhyay, P. Mosquito repellents: An insight into the chronological perspectives and novel discoveries. Acta Trop. 2017, 167, 216–230. [Google Scholar] [CrossRef]
- Guala, M.S.; Lapissonde, M.O.; Elder, H.V.; Pérez, G.A. Efecto acaricida del aceite esencial de aguaribay (Schinus molle L.) y sus fracciones en colmenares de abejas (Apis mellifera) en relación con la composición química. Inf. Tecnol. 2014, 25, 151–156. [Google Scholar] [CrossRef]
- Torres, F.C.; Machado Lucas, A.; Sardá-Ribeiro, V.L.; Martins, J.R.; von Poser, G.; Guala, M.S.; Elder, H.V.; Cassel, E. Influence of essential oil fractionation by vacuum distillation on acaricidal activity against the cattle tick. Braz. Arch. Biol. Technol. 2012, 55, 613–621. [Google Scholar] [CrossRef]
- Deveci, O.; Sukan, A.; Tuzun, N.; Hames Kocabas, E.E. Chemical composition, repellent and antimicrobial activity of Schinus molle L. J. Med. Plants Res. 2010, 4, 2211–2216. [Google Scholar]
- Belhoussaine, O.; El Kourchi, C.; Harhar, H.; Bouyahya, A.; El Yadini, A.; Fozia, F.; Alotaibi, A.; Ullah, R.; Tabyaoui, M. Chemical composition, antioxidant, insecticidal activity, and comparative analysis of essential oils of leaves and fruits of Schinus molle and Schinus terebinthifolius. Evid. Based Complement. Alternat. Med. 2022, 2022, 4288890. [Google Scholar] [CrossRef] [PubMed]
- Alba González, A.; Bonilla Rivera, P.; Arroyo Acevedo, J. Actividad cicatrizante de una pomada con aceite esencial de Schinus molle L. “molle” en ganado vacuno con heridas infectadas y en ratones. Cienc. Investig. 2009, 12, 29–36. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic functional ingredients from botanical sources for anti-pollution skincare products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Barrachina, L.M.D.; Bello, R.; Martínez-Cuesta, M.A.; Primo-Yúfera, E.; Esplunges, J. Analgesic and central depressor effects of the dichloromethanol extract from Schinus molle. Phytother. Res. 1998, 11, 317–319. [Google Scholar] [CrossRef]
- Hamdan, D.I.; Amal, A.; Al-Gendy, A.A.; El-Shazly, A.M. Chemical Composition and Cytotoxic Activity of the Essential Oils of Schinus molle Growing in Egypt. J. Pharm. Sci. Res. 2016, 8, 779–793. [Google Scholar]
- Tamarit-Urias, J.C. Determinación de los índices de calidad de pulpa para papel de 132 maderas latifoliadas. Madera Bosques 1996, 2, 29–41. [Google Scholar] [CrossRef]
- Mejía-Díaz, L.A.; Rutiaga-Quiñones, J.G. Chemical composition of Schinus molle L. wood and kraft pulping process. Rev. Mex. Ing. Quím. 2008, 7, 145–149. [Google Scholar]
- Olvera-Licona, G.; Machuca, R.; Borja, A.; Corona, A.; Zaragoza, I.; Arreola, J.G.; Jiménez, J. Xilotecnia de la madera de Schinus molle L. de una plantación forestal comercial en Hidalgo, México. Madera Bosques 2021, 27, e2711567. [Google Scholar] [CrossRef]
- Guerra-Coss, F.A.; Badano, E.I.; Cedillo-Rodríguez, I.E.; Ramírez-Albores, J.E.; Flores, J.; Barragán-Torres, F.; Flores-Cano, J.A. Modelling and validation of the spatial distribution of suitable habitats for the recruitment of invasive plants on climate change scenarios: An approach from the regeneration niche. Sci. Total Environ. 2021, 777, 146007. [Google Scholar] [CrossRef] [PubMed]
- Iponga, D.M.; Milton, S.J.; Richardson, D.M. Superiority in competition for light: A crucial attribute defining the impact of the invasive alien tree Schinus molle (Anacardiaceae) in South African savanna. J. Arid Environ. 2008, 72, 612–623. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Spencer, C.M. Miglitol: A review of its therapeutic potential in type 2 diabetes mellitus. Drugs 2000, 59, 521–549. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Mufti, A.; Caravaca, A.M.G.; Contreras, M.D.M.; Taamalli, A.; Carretero, A.S.; Aldawood, N.; Nahdi, S.; Alwasel, S.; et al. HPLC-ESI-QTOF-MS/MS profiling and therapeutic effects of Schinus terebinthifolius and Schinus molle fruits: Investigation of their antioxidant, antidiabetic, anti-inflammatory and antinociceptive properties. Inflammopharmacology 2021, 29, 467–481. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Hamed, M.; Sila, A.; Nahdi, S.; Alwasel, S.; Harrath, A.H.; Tlili, N. Multidirectional insights on polysaccharides from Schinus terebinthifolius and Schinus molle fruits: Physicochemical and functional profiles, in vitro antioxidant, anti-genotoxicity, antidiabetic, and antihemolytic capacities, and in vivo anti-inflammatory and anti-nociceptive properties. Int. J. Biol. Macromol. 2020, 15, 2576–2587. [Google Scholar]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- İlgün, S.; Şeker Karatoprak, G.; Çiçek Polat, D.; Köngül Şafak, E.; Yücel, Ç.; İnce, U.; Uvat, H.Ö.; Küpeli Akkol, E. Assessment of Phenolic Composition, Antioxidant Potential, Antimicrobial Properties, and Antidiabetic Activity in Extracts Obtained from Schinus molle L. Leaves and Fruits. Front. Biosci. (Landmark Ed.) 2023, 28, 353. [Google Scholar] [CrossRef]
- Kaabi, Y.A. Potential Roles of Anti-Inflammatory Plant-Derived Bioactive Compounds Targeting Inflammation in Microvascular Complications of Diabetes. Molecules 2022, 27, 7352. [Google Scholar] [CrossRef]
- Pannucci, E.; Spagnuolo, L.; De Gara, L.; Santi, L.; Dugo, L. Phenolic Compounds as Preventive and Therapeutic Agents in Diabetes-Related Oxidative Stress, Inflammation, Advanced Glycation End-Products Production and Insulin Sensitivity. Discov. Med. 2023, 35, 715–732. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Oyedeji, O.O.; Aremu, O.; Oyemitan, I.; Gwebu, E.T.; Oyedeji, A.O.; Nkeh-Chungag, B.N. Assessment of the analgesic, anti-inflammatory and sedative effects of the dichloromethanol extract of Schinus molle. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 372–380. [Google Scholar] [PubMed]
- Shawa, A.; Nayal, R.; Abajy, M.Y.; Hasan, N.K. In vitro and In vivo Assessment of the Anti-inflammatory activity of Schinus molle. RJPT 2025, 18, 2049–2054. [Google Scholar] [CrossRef]
- Yueqin, Z.; Recio, M.C.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.L. Isolation of two triterpenoids and a biflavanone with anti-Inflammatory activity from Schinus molle fruits. Planta Med. 2003, 69, 893–898. [Google Scholar]
- Nicolls, M.R.; Haskins, K.; Flores, S.C. Oxidant stress, immune dysregulation, and vascular function in type I diabetes. Antioxid. Redox Signal 2007, 9, 879–889. [Google Scholar] [CrossRef]
- Davì, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal 2005, 7, 256–268. [Google Scholar] [CrossRef]
- Bashkin, A.; Ghanim, M.; Abu-Farich, B.; Rayan, M.; Miari, R.; Srouji, S.; Rayan, A.; Falah, M. Forty-One Plant Extracts Screened for Dual Antidiabetic and Antioxidant Functions: Evaluating the Types of Correlation between α-Amylase Inhibition and Free Radical Scavenging. Molecules 2021, 26, 317. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, D.W.; Kim, J.G.; Shin, Y.; Jung, S.K.; Kim, Y.J. Analysis of the Chemical, Antioxidant, and Anti-Inflammatory Properties of Pink Pepper (Schinus molle L.). Antioxidants 2021, 30, 1062. [Google Scholar] [CrossRef]
- do Rosário Martins, M.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Zayed, M.Z.; Ali, H.M. Chemical composition, antioxidant and antibacterial activities of extracts from Schinus molle wood branch growing in Egypt. J. Wood Sci. 2016, 62, 548–561. [Google Scholar] [CrossRef]
- Shehata, M.E.; El-Sherbiny, G.M.; Sharaf, M.H.; Kalaba, M.H.; Shaban, A.S. Phytochemical analysis, antimicrobial, antioxidant, and cytotoxicity activities of Schinus molle (L.) extracts. Biomass Convers. Biorefinery 2025, 15, 3753–3770. [Google Scholar]
- Bras, C.; Gumilar, F.; Gandini, N.; Minetti, A.; Ferrero, A. Evaluation of the acute dermal exposure of the ethanolic and hexanic extracts from leaves of Schinus molle var. areira L. in rats. J. Ethnopharmacol. 2011, 137, 1450–1456. [Google Scholar] [CrossRef]
- Machado, C.D.; Farago, P.V.; Costa, C.M.; Farias, K.S.; Silva, D.B.; Marques, A.A.M.; Moreno, K.G.T.; Pael, L.A.B.; da Silva, M.L.F.; Gasparotto Junior, A.; et al. Acute toxicity and genotoxicity of Schinus molle L. aqueous extract/ethanol-soluble fraction in rats. J. Ethnopharmacol. 2024, 333, 118499. [Google Scholar] [CrossRef]
- Bras, C.; Domínguez, S.; Codón, S.; Minetti, A.; Ferrero, A. Consequences of subchronic exposure to ethanolic extract from fruits and leaves of Schinus molle var. areira L. in mice. J. Ethnopharmacol. 2010, 132, 321–327. [Google Scholar] [CrossRef]
- Ferrero, A.; Minetti, A.; Bras, C.; Zanetti, N. Acute and subacute toxicity evaluation of ethanolic extract from fruits of Schinus molle in rats. J. Ethnopharmacol. 2007, 113, 441–447. [Google Scholar] [CrossRef]
- Mügge, F.L.B.; Morlock, G.E. Chemical and cytotoxicity profiles of 11 pink pepper (Schinus spp.) samples via non-targeted hyphenated high-performance thin-layer chromatography. Metabolomics 2023, 19, 48. [Google Scholar] [CrossRef]
- Rios, M.Y.; Salinas, D.; Villarreal, M.L. Cytotoxic activity of moronic acid and identification of the new triterpene 3,4-seco-olean-18-ene-3,28-dioic acid from Phoradendron reichenbachianum. Planta Med. 2001, 67, 443–446. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Li, W.; Yuan, G.; Pan, Y.; Wang, C.; Chen, H. Network Pharmacology Studies on the Bioactive Compounds and Action Mechanisms of Natural Products for the Treatment of Diabetes Mellitus: A Review. Front Pharmacol. 2017, 23, 74. [Google Scholar] [CrossRef]
- Riyaphan, J.; Pham, D.C.; Leong, M.K.; Weng, C.F. In Silico Approaches to Identify Polyphenol Compounds as α-Glucosidase and α-Amylase Inhibitors against Type-II Diabetes. Biomolecules 2021, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ryu, Y.B.; Kang, N.S.; Lee, B.W.; Heo, J.S.; Jeong, I.Y.; Park, K.H. Glycosidase inhibitory flavonoids from Sophora flavescens. Biol Pharm Bull. 2006, 29, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Overview of oxidative stress and inflammation in diabetes. J Diabetes 2024, 16, e70014. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Nkhumeleni, Z.; Phoswa, W.N.; Mokgalaboni, K. Purslane Ameliorates Inflammation and Oxidative Stress in Diabetes Mellitus: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12276. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Aldahmash, W.; Mnafgui, K.; Hichem, A.; Gómez-Caravaca, A.M.; Del Mar Contreras, M.; Taamalli, A.; Alwasel, S.; Segura-Carretero, A.; et al. In vivo evaluation and molecular docking studies of Schinus molle L. fruit extract protective effect against isoproterenol-induced infarction in rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 80910–80925. [Google Scholar] [CrossRef]
- Morieri, M.L.; Pipino, C.; Doria, A. Pharmacogenetics of Cardiovascular Prevention in Diabetes: From Precision Medicine to Identification of Novel Targets. J. Pers. Med. 2022, 12, 1402. [Google Scholar] [CrossRef]
- Vuorinen, A.; Seibert, J.; Papageorgiou, V.P.; Rollinger, J.M.; Odermatt, A.; Schuster, D.; Assimopoulou, A.N. Pistacia lentiscus Oleoresin: Virtual Screening and Identification of Masticadienonic and Isomasticadienonic Acids as Inhibitors of 11β-Hydroxysteroid Dehydrogenase 1. Planta Med. 2015, 81, 525–532. [Google Scholar] [CrossRef]
- Eryigit, T.; Yildirim, B.; Ekici, K.; Çirka, M. Chemical Composition, Antimicrobial and Antioxidant Properties of Schinus molle L. Essential Oil from Turkey. JEOBP 2017, 20, 570–577. [Google Scholar]
- Floris, S.; Di Petrillo, A.; Pintus, F.; Delogu, G.L. Pistacia lentiscus: Phytochemistry and Antidiabetic Properties. Nutrients 2024, 16, 1638. [Google Scholar] [CrossRef]
- Pett, K.D.; Alex, P.G.; Weisfuss, C.; Sandhu, A.; Burton-Freeman, B.; Edirisinghe, I. Mango Consumption Is Associated with Increased Insulin Sensitivity in Participants with Overweight/Obesity and Chronic Low-Grade Inflammation. Nutrients 2025, 17, 490. [Google Scholar] [CrossRef]
- Rossini, C.; Menéndez, P.; Dellacassa, E.; Moyna, P. Essential oils from leaves of Schinus molle and S. lentiscifolius of Uruguayan origin. J. Essent. Oil Res. 1996, 8, 71–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, R.M.; Huerta-Reyes, M. Antidiabetic Properties of the Tropical Tree Schinus molle L. (pirul): A Comprehensive Review. Pharmaceuticals 2025, 18, 1661. https://doi.org/10.3390/ph18111661
Fonseca RM, Huerta-Reyes M. Antidiabetic Properties of the Tropical Tree Schinus molle L. (pirul): A Comprehensive Review. Pharmaceuticals. 2025; 18(11):1661. https://doi.org/10.3390/ph18111661
Chicago/Turabian StyleFonseca, Rosa María, and Maira Huerta-Reyes. 2025. "Antidiabetic Properties of the Tropical Tree Schinus molle L. (pirul): A Comprehensive Review" Pharmaceuticals 18, no. 11: 1661. https://doi.org/10.3390/ph18111661
APA StyleFonseca, R. M., & Huerta-Reyes, M. (2025). Antidiabetic Properties of the Tropical Tree Schinus molle L. (pirul): A Comprehensive Review. Pharmaceuticals, 18(11), 1661. https://doi.org/10.3390/ph18111661






