Additive Anticancer and Antioxidant Effects of Metformin and Luteolin in Breast and Colorectal Cancer Cell Lines
Abstract
1. Introduction
2. Results
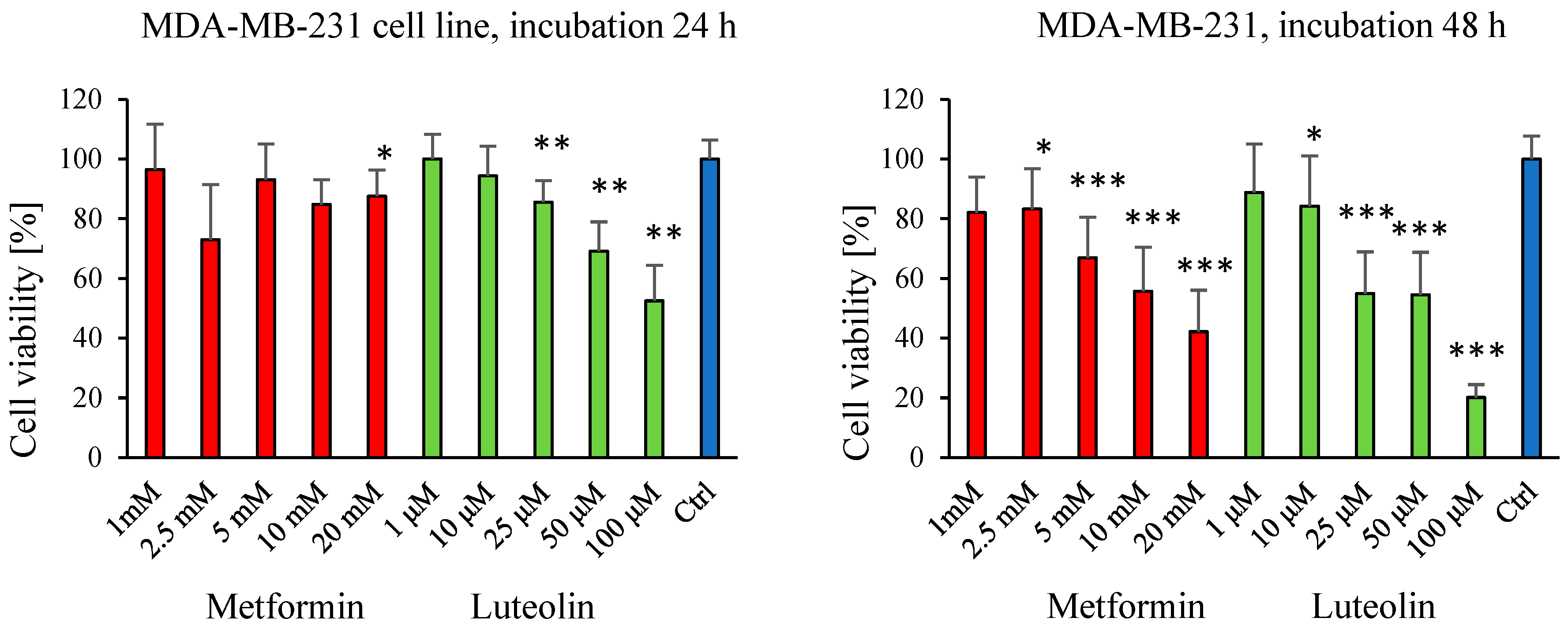
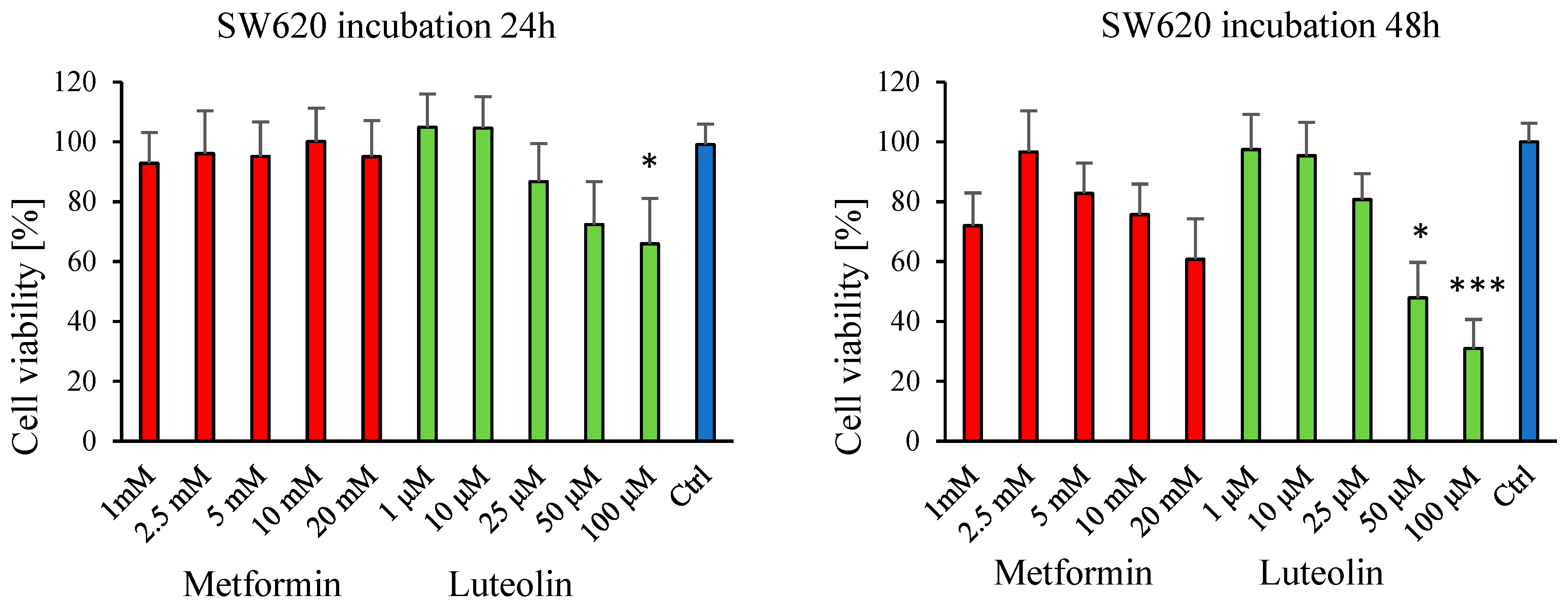
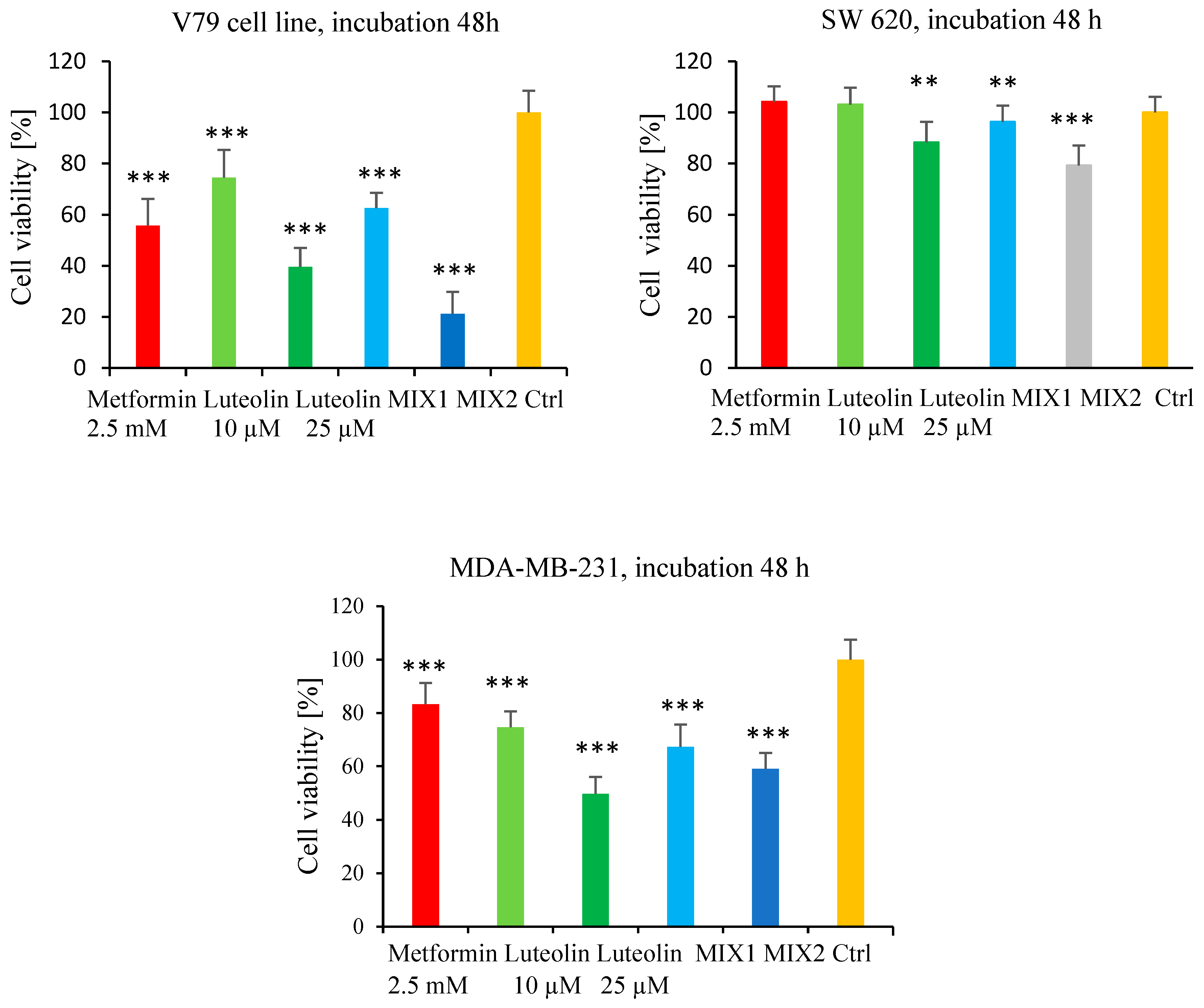
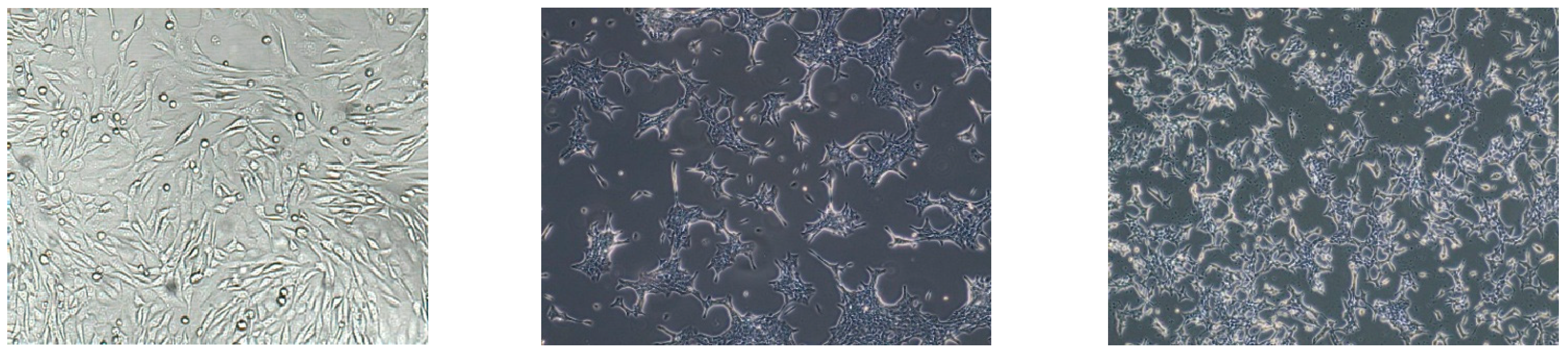
2.1. Effect of Luteolin and Metformin on Viability of SW620, MDA-MB-231, and V79 Cells
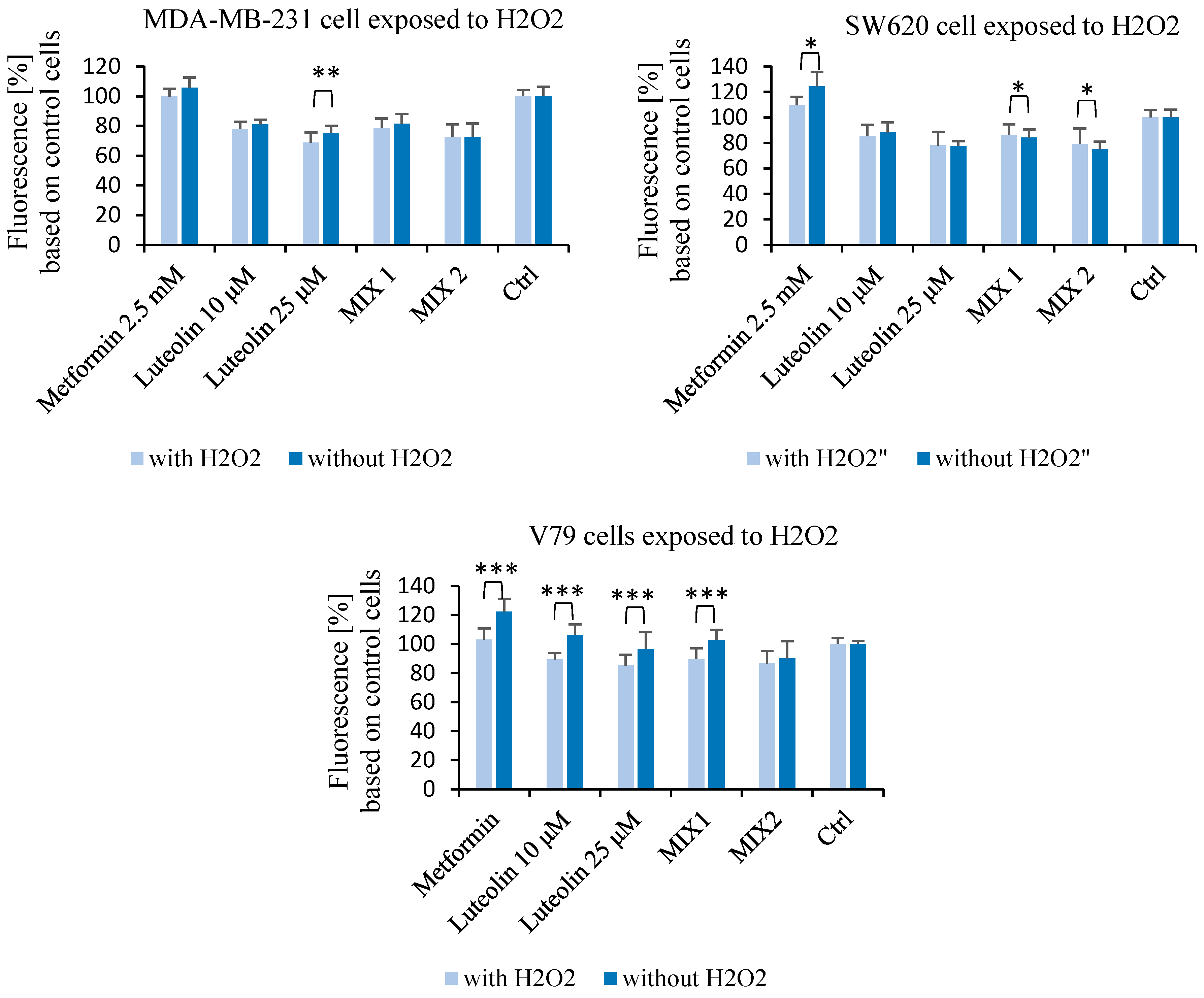
2.2. Effect of Luteolin and Metformin Under Oxidative Stress Conditions
2.3. Metabolomics Analysis
Identification of Intracellular and Extracellular Metabolites in V79, MDA-MB-231, and SW620 Cell Lines
2.4. Multivariate Data Analysis
Intracellular and Extracellular Metabolism
2.5. Metabolite Relative Concentrations and Statistical Analysis
2.5.1. Intracellular Metabolites
2.5.2. Extracellular Metabolites
2.6. Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Assessment of Cytotoxicity and Reactive Oxygen Species Levels
4.2.1. Cell Viability Assay—MTT Test
4.2.2. Measurement of Reactive Oxygen Species—DCF-DA Assay
4.2.3. Statistical Analysis of Biological Activity Data
4.3. NMR Analysis
4.3.1. Sample Preparation and 1H NMR Measurement
4.3.2. Sonication and Evaporation of Samples
4.3.3. 1H NMR Spectroscopy Analysis
4.3.4. Univariate Processing and Statistical Data Analysis of 1D NMR Spectra
4.3.5. Multivariate Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr.-Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef]
- Morales, D.R.; Morris, A.D. Metformin in Cancer Treatment and Prevention. Rev. Med. 2015, 66, 17–29. [Google Scholar] [CrossRef]
- Nestler, J.E.; Strauss, J.F., 3rd. Insulin as an Effector of Human Ovarian and Adrenal Steroid Metabolism. Endocrinol. Metab. Clin. North Am. 1991, 20, 807–823. [Google Scholar] [CrossRef]
- Key, T.J.; Allen, N.E. Hormones and breast cancer. IARC Sci. Publ. 2002, 156, 273–276. [Google Scholar]
- Zhang, Y.; Guan, M.; Zheng, Z.; Zhang, Q.; Gao, F.; Xue, Y. Effects of Metformin on CD133+ Colorectal Cancer Cells in Diabetic Patients. PLoS ONE 2013, 8, e81264. [Google Scholar] [CrossRef]
- Gonda, T.A.; Tu, S.; Wang, T.C. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle 2009, 8, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.M.; Galal, M.A.; Ain-Shoka, A.A.; Shouman, S.A. Combination of metformin and 5-aminosalicylic acid cooperates to decrease proliferation and induce apoptosis in colorectal cancer cell lines. BMC Cancer 2016, 16, 126. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2020, 42, 77–96. [Google Scholar] [CrossRef]
- Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. Int. J. Mol. Sci. 2024, 25, 4083. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and Cancer Hallmarks: Shedding New Lights on Therapeutic Repurposing. J. Transl. Med. 2023, 21, 403. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Rehman, A.; Satyam, S.M.; El-Tanani, M.; Prabhakar, S.; Kumari, R.; Shetty, P.; Mohammed, S.S.N.; Nafees, Z.; Alomar, B. Metformin Beyond Diabetes: A Precision Gerotherapeutic and Immunometabolic Adjuvant for Aging and Cancer. Cancers 2025, 17, 2466. [Google Scholar] [CrossRef] [PubMed]
- Mahfauz, M.; Yuruker, O.; Kalkan, R. Repurposing metformin as a potential anticancer agent using in silico technique. DARU J. Pharm. Sci. 2024, 32, 549–555. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Gupta, R.; Kumar, D.; Bhattacharya, S.; Sarkar, A.; Kumar, P. Can luteolin be a therapeutic molecule for both colon cancer and diabetes? Briefings Funct. Genom. 2019, 18, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yılmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kılıç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on In Vivo Pharmacological Effects and Bioavailability Traits. Oxid. Med. Cell. Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Uma, D.P.; Satyamitra, M. Protection against prenatal irradiation-induced genomic instability and its consequences in adult mice by Ocimum flavonoids, orientin and vicenin. Int. J. Radiat. Biol. 2004, 80, 653–662. [Google Scholar]
- Devi, P.U.; Ganasoundari, A.; Vrinda, B.; Srinivasan, K.K.; Unnikrishnan, M.K. Radiation Protection by the Ocimum Flavonoids Orientin and Vicenin: Mechanisms of Action. Radiat. Res. 2000, 154, 455–460. [Google Scholar] [CrossRef]
- Nayak, V.; Devi, P.U. Protection of Mouse Bone Marrow against Radiation-Induced Chromosome Damage and Stem Cell Death by the Ocimum Flavonoids Orientin and Vicenin. Radiat. Res. 2005, 163, 165–171. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-J.; Song, Z.-H.; Lin, H.-H.; Han, X.-F.; Zhang, S.; Yang, Y.-M. Luteolin as a glycolysis inhibitor offers superior efficacy and lesser toxicity of doxorubicin in breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 372, 497–502. [Google Scholar] [CrossRef]
- Bagli, E.; Stefaniotou, M.; Morbidelli, L.; Ziche, M.; Psillas, K.; Murphy, C.; Fotsis, T. Luteolin Inhibits Vascular Endothelial Growth Factor-Induced Angiogenesis; Inhibition of Endothelial Cell Survival and Proliferation by Targeting Phosphatidylinositol 3′-Kinase Activity. Cancer Res. 2004, 64, 7936–7946. [Google Scholar] [CrossRef]
- Han, Y.; Xiao, Y.; Yu, L.; Chen, J.; Yang, X.; Cui, H.; Liang, J. Advances in the Mechanism of Luteolin against Hepatocellular Carcinoma Based on Bioinformatics and Network Pharmacology. J. Cancer 2023, 14, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.; Lin, Y. A Critical Role of Luteolin-Induced Reactive Oxygen Species in Blockage of Tumor Necrosis Factor-Activated Nuclear Factor-κB Pathway and Sensitization of Apoptosis in Lung Cancer Cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar] [CrossRef]
- Shi, R.; Huang, Q.; Zhu, X.; Ong, Y.-B.; Zhao, B.; Lu, J.; Ong, C.-N.; Shen, H.-M. Luteolin sensitizes the anticancer effect of cisplatin via c-Jun NH2-terminal kinase–mediated p53 phosphorylation and stabilization. Mol. Cancer Ther. 2007, 6, 1338–1347. [Google Scholar] [CrossRef]
- Kim, H.W. Metabolomic Approaches to Investigate the Effect of Metformin: An Overview. Int. J. Mol. Sci. 2021, 22, 10275. [Google Scholar] [CrossRef] [PubMed]
- Ser, Z.; Gao, X.; Johnson, C.; Mehrmohamadi, M.; Liu, X.; Li, S.; Locasale, J.W. Targeting One Carbon Metabolism with an Antimetabolite Disrupts Pyrimidine Homeostasis and Induces Nucleotide Overflow. Cell Rep. 2016, 15, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Karekar, A.K.; Dandekar, S.P. Cancer metabolomics: A tool of clinical utility for early diagnosis of gynaecological cancers. Indian J. Med. Res. 2021, 154, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Lin, J.; Liu, Y.-E.; Chen, H.-F.; Hsu, K.-W.; Lin, S.-H.; Peng, K.-Y.; Lin, K.-J.; Hsieh, C.-C.; Chen, D.-R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gong, F.; Liu, P.; Miao, Q. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene 2018, 664, 50–57. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.; Wang, X.; Kang, L.; Yang, Y.; Liu, Y.; Yang, L.; Li, J.; Yang, L.; Liu, J.; et al. Metformin potentiates anti-tumor effect of resveratrol on pancreatic cancer by down-regulation of VEGF-B signaling pathway. Oncotarget 2016, 7, 84190–84200. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, C.; Lu, Q.; Cao, Y.; Yu, W.; Li, W.; Liu, B.; Gao, X.; Lü, J.; Pan, X. Metformin inhibits the tumor-promoting effect of low-dose resveratrol, and enhances the anti-tumor activity of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic. Biol. Med. 2022, 180, 108–120. [Google Scholar] [CrossRef]
- Cabello, P.; Pineda, B.; Tormo, E.; Lluch, A.; Eroles, P. The Antitumor Effect of Metformin Is Mediated by miR-26a in Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1298. [Google Scholar] [CrossRef]
- Mogavero, A.; Maiorana, M.V.; Zanutto, S.; Varinelli, L.; Bozzi, F.; Belfiore, A.; Volpi, C.C.; Gloghini, A.; Pierotti, M.A.; Gariboldi, M. Metformin transiently inhibits colorectal cancer cell proliferation as a result of either AMPK activation or increased ROS production. Sci. Rep. 2017, 7, 15992. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef]
- Lo, S.; Leung, E.; Fedrizzi, B.; Barker, D. Syntheses of mono-acylated luteolin derivatives, evaluation of their antiproliferative and radical scavenging activities and implications on their oral bioavailability. Sci. Rep. 2021, 11, 12595. [Google Scholar] [CrossRef]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Xu, Y.; Yang, J.; Fang, Y.; Lou, H.; Han, W.; Zhang, M.; Chen, W.; Wang, K.; Li, D.; et al. Use of Metformin Alone Is Not Associated with Survival Outcomes of Colorectal Cancer Cell but AMPK Activator AICAR Sensitizes Anticancer Effect of 5-Fluorouracil through AMPK Activation. PLoS ONE 2014, 9, e97781. [Google Scholar] [CrossRef]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.M.; Pellegrini, F.; Nicolucci, A. Metformin Therapy and Risk of Cancer in Patients with Type 2 Diabetes: Systematic Review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef]
- Zhang, Y.; Storr, S.J.; Johnson, K.; Green, A.R.; Rakha, E.A.; Ellis, I.O.; Morgan, D.A.; Martin, S.G. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget 2014, 5, 12936–12949. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.-C.; Ku, J.-L. Metformin increases chemo-sensitivity via gene downregulation encoding DNA replication proteins in 5-Fu resistant colorectal cancer cells. Oncotarget 2017, 8, 56546–56557. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Yu, Y.; Vasudevan, A.; Farhana, L.; Rajendra, S.G.; Levi, E.; Majumdar, A.P.N. Metformin: A Potential Therapeutic Agent for Recurrent Colon Cancer. PLoS ONE 2014, 9, e84369. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chuang, T.-W.; Chen, L.-J.; Niu, H.-S.; Chung, K.-M.; Cheng, J.-T.; Lin, K.-C. Increase in apoptosis by combination of metformin with silibinin in human colorectal cancer cells. World J. Gastroenterol. 2015, 21, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, S.; Hernadez-Aya, L.F.; Lei, X.; Meric-Bernstam, F.; Litton, J.K.; Hsu, L.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer 2012, 118, 1202–1211. [Google Scholar] [CrossRef]
- Yan, Y.; Jun, C.; Lu, Y.; Jiangmei, S. Combination of metformin and luteolin synergistically protects carbon tetrachloride-induced hepatotoxicity: Mechanism involves antioxidant, anti-inflammatory, antiapoptotic, and Nrf2/HO-1 signaling pathway. BioFactors 2019, 45, 598–606. [Google Scholar] [CrossRef]
- Biswas, S.; Chida, A.S.; Rahman, I. Redox modifications of protein–thiols: Emerging roles in cell signaling. Biochem. Pharmacol. 2006, 71, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-Ros Crosstalk in the Control of Cell Death and Aging. J. Signal Transduct. 2012, 2012, 329635. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, C.; Huo, K.; Wang, Q.; Lu, L.; Zhang, Q.; Wang, Y.; Wang, W. Luteolin Prevents H2O2-Induced Apoptosis in H9C2 Cells through Modulating Akt-P53/Mdm2 Signaling Pathway. BioMed Res. Int. 2016, 2016, 5125836. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Hu, B.; Feng, G.; Xiang, M.; Deng, Y.; Tan, M.; Li, J.; Song, J. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 2020, 21, 13–27. [Google Scholar] [CrossRef]
- Liu, W.; Qaed, E.; Zhu, H.G.; Dong, M.X.; Tang, Z. Non-energy mechanism of phosphocreatine on the protection of cell survival. Biomed. Pharmacother. 2021, 141, 111839. [Google Scholar] [CrossRef]
- Oizel, K.; Tait-Mulder, J.; Fernandez-De-Cossio-Diaz, J.; Pietzke, M.; Brunton, H.; Lilla, S.; Dhayade, S.; Athineos, D.; Blanco, G.R.; Sumpton, D.; et al. Formate induces a metabolic switch in nucleotide and energy metabolism. Cell Death Dis. 2020, 11, 310. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Królik, P.W.; Rusinek, B.; Dobrzańska-Pielech, T.; Rudnicka-Drożak, E. Metformin -associated lactic acidosis. Gieriatrics Clin. Probl. 2018, 12, 117–125. [Google Scholar]
- Guimaraes-Ferreir, L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein 2014, 12, 126–131. [Google Scholar] [CrossRef]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, B.; Zhao, A.; Qiu, Y.; Zhao, X.; Garmire, L.; Shvetsov, Y.B.; Yu, H.; Yen, Y.; Jia, W. Lowered circulating aspartate is a metabolic feature of human breast cancer. Oncotarget 2015, 6, 33369–33381. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Richard, V.; Conotte, R.; Mayne, D.; Colet, J.-M. Does the 1H-NMR plasma metabolome reflect the host-tumor interactions in human breast cancer? Oncotarget 2017, 8, 49915–49930. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.; Viollet, B.; Caton, P.; Leclerc, J.; Sakakibara, I.; Foretz, M.; Holness, M.; Sugden, M. The AMPK-SIRT signaling network regulates glucose tolerance under calorie restriction conditions. Life Sci. 2014, 100, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Kreuzaler, P.; Panina, Y.; Segal, J.; Yuneva, M. Adapt and conquer: Metabolic flexibility in cancer growth, invasion and evasion. Mol. Metab. 2020, 33, 83–101. [Google Scholar] [CrossRef]
- Kostidis, S.; Addie, R.D.; Morreau, H.; Mayboroda, O.A.; Giera, M. Quantitative NMR analysis of intra- and extracellular metabolism of mammalian cells: A tutorial. Anal. Chim. Acta 2017, 980, 1–24. [Google Scholar] [CrossRef]

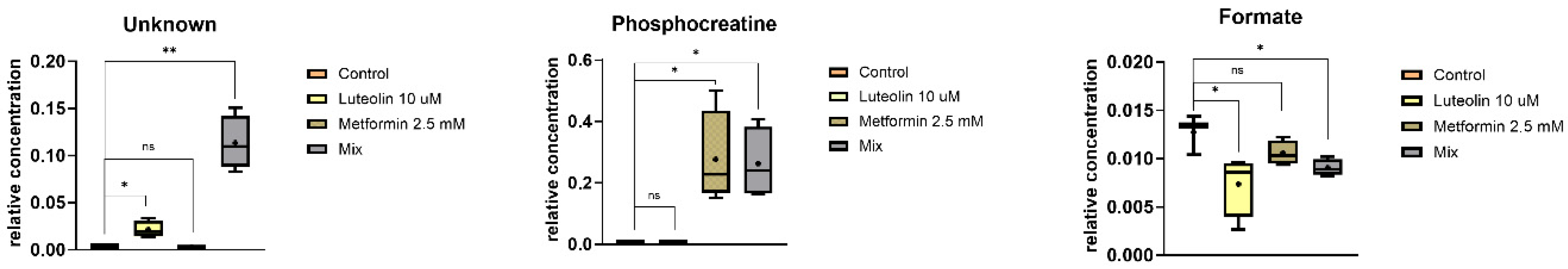


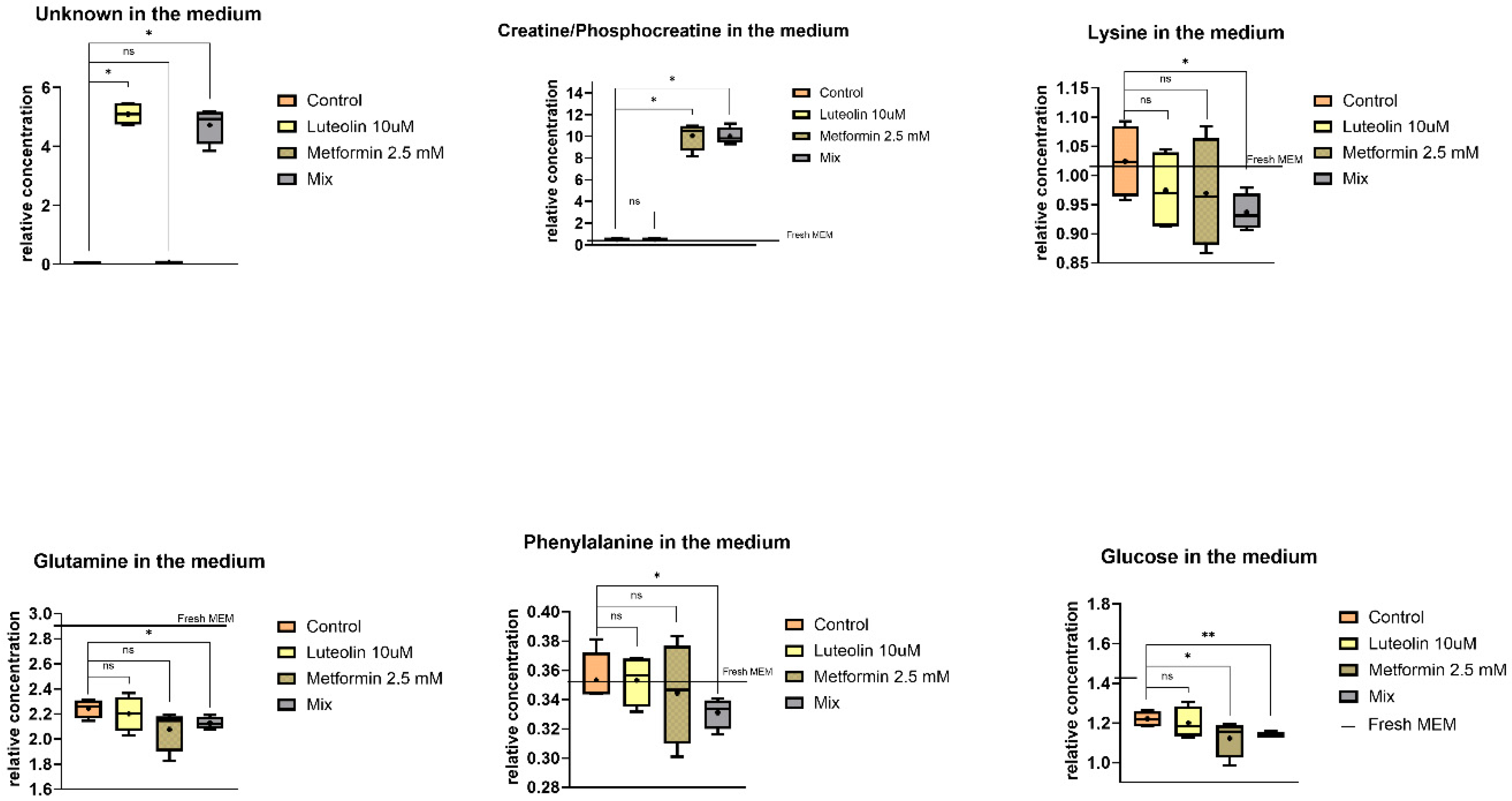
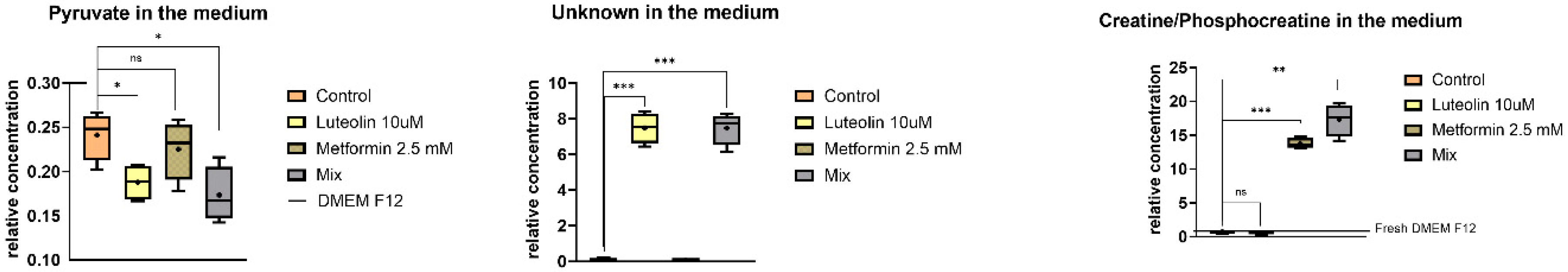
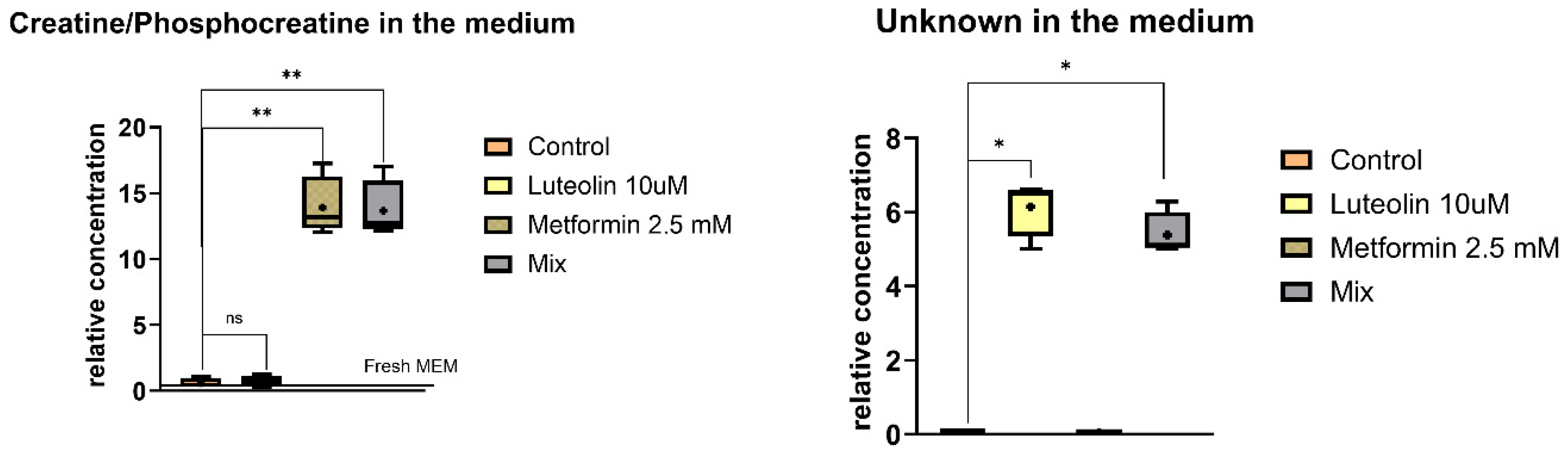

| Pathway Name | Hits | Raw p | Holm p | FDR |
|---|---|---|---|---|
| Arginine and Proline Metabolism | 5 | 3.12 × 10−7 | 3.06 × 10−5 | 3.06 × 10−5 |
| Urea Cycle | 4 | 2.40 × 10−6 | 2.33 × 10−4 | 1.85 × 10−3 |
| Mitochondrial Electron Transport Chain | 3 | 5.66 × 10−5 | 5.43 × 10−3 | 1.85 × 10−3 |
| Purine Metabolism | 4 | 1.23 × 10−4 | 1.17 × 10−2 | 3.02 × 10−3 |
| Ammonia Recycling | 3 | 2.58 × 10−4 | 2.42 × 10−2 | 4.64 × 10−3 |
| Citric Acid Cycle | 3 | 2.84 × 10−4 | 2.64 × 10−2 | 4.64 × 10−3 |
| Aspartate Metabolism | 3 | 3.73 × 10−4 | 3.43 × 10−2 | 5.22 × 10−3 |
| Pathway Name | Hits | Raw p | −log(p) | Holm Adjust | FDR | Impact |
|---|---|---|---|---|---|---|
| Arginine biosynthesis | 2 | 7.23 × 10−4 | 3.14 × 100 | 5.78 × 10−2 | 5.78 × 10−2 | 0.01 |
| Alanine, aspartate and glutamate metabolism | 2 | 2.95 × 10−3 | 2.53 × 100 | 2.33 × 10−1 | 1.18 × 10−1 | 0.23 |
| Purine metabolism | 2 | 1.79 × 10−2 | 1.75 × 100 | 1.00 × 100 | 4.76 × 10−1 | 0.03 |
| Nicotinate and nicotinamide metabolism | 1 | 4.68 × 10−2 | 1.33 × 100 | 1.00 × 100 | 6.31 × 10−1 | 0.03 |
| Histidine metabolism | 1 | 4.98 × 10−2 | 1.33 × 100 | 1.00 × 100 | 6.31 × 10−1 | 0.03 |
| Pantothenate and CoA biosynthesis | 1 | 6.20 × 10−2 | 1.21 × 100 | 1.00 × 100 | 6.31 × 10−1 | 0.03 |
| Citrate cycle (TCA cycle) | 1 | 6.20 × 10−2 | 1.21 × 100 | 1.00 × 100 | 6.31 × 10−1 | 0.03 |
| Beta-Alanine metabolism | 1 | 7.10 × 10−2 | 1.15 × 100 | 1.00 × 100 | 6.31 × 10−1 | 0.03 |
| Pyruvate metabolism | 1 | 7.10 × 10−2 | 1.15 × 100 | 1.00 × 100 | 6.31 × 10−1 | 0.03 |
| Glycine, serine and threonine metabolism | 1 | 1.09 × 10−1 | 9.82 × 10−1 | 1.00 × 100 | 7.95 × 10−1 | 0.02 |
| Arginine and proline metabolism | 1 | 1.09 × 10−1 | 9.82 × 10−1 | 1.00 × 100 | 7.95 × 10−1 | 0.02 |
| Tyrosine metabolism | 1 | 1.27 × 10−1 | 9.88 × 10−1 | 1.00 × 100 | 8.44 × 10−1 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gębczak, K.; Cwynar-Zając, Ł.; Sapeta-Nowińska, M.; Barg, E. Additive Anticancer and Antioxidant Effects of Metformin and Luteolin in Breast and Colorectal Cancer Cell Lines. Pharmaceuticals 2025, 18, 1660. https://doi.org/10.3390/ph18111660
Gębczak K, Cwynar-Zając Ł, Sapeta-Nowińska M, Barg E. Additive Anticancer and Antioxidant Effects of Metformin and Luteolin in Breast and Colorectal Cancer Cell Lines. Pharmaceuticals. 2025; 18(11):1660. https://doi.org/10.3390/ph18111660
Chicago/Turabian StyleGębczak, Katarzyna, Łucja Cwynar-Zając, Monika Sapeta-Nowińska, and Ewa Barg. 2025. "Additive Anticancer and Antioxidant Effects of Metformin and Luteolin in Breast and Colorectal Cancer Cell Lines" Pharmaceuticals 18, no. 11: 1660. https://doi.org/10.3390/ph18111660
APA StyleGębczak, K., Cwynar-Zając, Ł., Sapeta-Nowińska, M., & Barg, E. (2025). Additive Anticancer and Antioxidant Effects of Metformin and Luteolin in Breast and Colorectal Cancer Cell Lines. Pharmaceuticals, 18(11), 1660. https://doi.org/10.3390/ph18111660







