1. Introduction
Antibiotic resistance constitutes one of the greatest contemporary challenges to public health, impacting infection-related mortality, prolonging the course of diseases, increasing treatment costs, and reducing social productivity. In addition, it compromises the effectiveness of medical interventions that rely on antimicrobials, such as prophylactic surgeries and chemotherapy [
1,
2,
3,
4]. The emergence of resistance largely results from the adaptive capacity of bacterial populations, intensified by the indiscriminate use of antibiotics, which favors the acquisition and dissemination of resistance mechanisms.
Among multidrug-resistant pathogens,
Staphylococcus aureus (
S. aureus) stands out due to its high virulence, ability to evade immune responses, and increasing resistance to antimicrobials [
5,
6,
7,
8]. This versatile microorganism can cause anything from mild skin lesions to severe diseases such as sepsis, pneumonia, and toxic shock syndrome through the production of multiple virulence factors, including toxins, adhesion proteins, and immune evasion systems such as protein A, superantigenic toxins, and the agr quorum-sensing system [
9].
Resistance in
S. aureus arises both from spontaneous genetic mutations and from the acquisition of resistance genes via horizontal transfer. Mobile genetic elements such as plasmids, transposons, pathogenic islands (SaPIs), and staphylococcal chromosomal cassettes (SCCs) enable the rapid dissemination of resistance determinants [
10]. Among the most important mechanisms are enzymatic inactivation of antibiotics, modification of molecular targets, reduced membrane permeability, and activation of efflux pumps [
11,
12,
13]. Processes such as conjugation, natural transformation, and phage-mediated transduction contribute to the emergence of highly virulent and resistant strains.
Among these mechanisms, efflux pumps stand out as central resistance systems, promoting the active extrusion of antibiotics and other toxic compounds [
14]. Studies indicate that the
S. aureus genome encodes at least 31 multidrug efflux pumps distributed among the major transporter families [
15]. The Major Facilitator Superfamily (MFS) has received particular attention, especially the NorA pump, responsible for the extrusion of fluoroquinolones and other drugs. Its function operates through a proton/drug antiport mechanism, which may be constitutively expressed or induced by exposure to antimicrobials [
16,
17,
18].
Given the scarcity of new antimicrobials, alternative strategies have been explored, including the use of efflux pump inhibitors (EPIs) [
19]. These compounds block drug extrusion, potentially reversing efflux-mediated resistance, reducing required doses, minimizing adverse effects, and restoring the efficacy of previously ineffective antibiotics [
19,
20,
21,
22]. EPIs can be natural or synthetic, and compounds derived from medicinal plants, such as chalcones, have shown promise due to their structural diversity and broad spectrum of biological activities [
21,
23].
The literature reports various efflux pump inhibitors (EPIs), both synthetic and natural, capable of restoring antibiotic effectiveness against resistant strains. Among the synthetic inhibitors, PAβN (phenylalanyl-arginyl-β-naphthylamide) is notable, functioning through competitive inhibition and gene regulation [
24,
25]; CCCP (carbonyl cyanide-m-chlorophenylhydrazone), which disrupts ATP synthesis [
26]; NMP (1-(1-naphthylmethyl)-piperazine), which hinders the proper assembly of pumps [
27]; and MBX2319, a pyrazolopyridine derivative with proven activity against
E. coli [
28]. In the natural inhibitors group, silybin stands out for reducing the expression of NorA and qacA/B genes in
S. aureus [
29]; boeravinone B, which enhances ciprofloxacin’s action [
30]; curcumin, an inhibitor of the TetK pump [
31]; berberine and columbamine, which interfere with ATP synthesis [
32]; and reserpine, which binds directly to transport proteins [
33]. Additionally, essential oils from Origanum vulgare and Nigella sativa have shown potential in modulating efflux gene expression and altering bacterial membrane permeability [
34,
35].
Although numerous natural and synthetic compounds have been described as efflux pump inhibitors, the search for effective, safe, and structurally versatile molecules still represents a significant challenge in combating bacterial resistance [
22]. In this context, chalcones stand out for their broad spectrum of biological activities and their ability to modulate resistance mechanisms [
36]. These molecules, widely distributed in flowers, leaves, fruits, and roots, can be obtained by chemical or semi-synthetic synthesis [
37], exhibit low toxicity, a characteristic yellow coloration, and act as precursors of various bioactive compounds [
38,
39]. Studies indicate that chalcones display antioxidant, anti-inflammatory [
40], antimicrobial [
39], anticancer [
38], and antiviral activities [
41], in addition to enhancing antibiotic action and reducing virulence factors such as biofilm formation and toxin production [
42,
43,
44].
However, investigations involving specific synthetic derivatives, such as the chalcone CMA4DMA, remain scarce. Thus, the present study aimed to evaluate the potential of the synthetic chalcone CMA4DMA as an inhibitor of the NorA efflux pump in S. aureus through an integrated in vitro and in silico approach, aiming to contribute to the development of new therapeutic strategies against resistant bacterial infections.
3. Discussion
The lack of a relevant MIC does not entirely disqualify a substance for potential application in other studies against microorganisms, particularly when considering its possible use as an antibiotic adjuvant. In the case of efflux pump inhibitors, the absence of direct activity may even enhance their applicability, as previously noted in the literature [
55].
Restoring the activity of antibiotics or other antibacterial agents through adjuvants is one of the main strategies currently employed to control strains harboring multiple mechanisms of resistance to antibacterial drugs or other bactericidal agents [
56]. This is particularly relevant because the discovery of new effective antibiotics is increasingly rare. Furthermore, even when a new antibiotic is discovered, the likelihood that its indiscriminate use will lead to bacterial resistance is high [
57]. Various methods exist to assess the ability of substances to restore antibiotic activity and, consequently, to identify the bacterial resistance mechanisms involved. Reduction in the minimum inhibitory concentration is one of the first analyses performed to indicate the restoration of antibiotic activity, typically using sub-inhibitory concentrations of the test substances in combination with an antibiotic or another antibacterial agent [
58].
In the present study, as shown in
Figure 1, CMA4DMA at sub-inhibitory concentrations was able to restore antibiotic activity by reducing the MIC, and the same effect was observed in combination with ethidium bromide. In the case of ethidium bromide, the MIC reduction is indicative of efflux pump inhibition, which is reinforced by the action of the positive control, the efflux pump inhibitor CCCP. It is also noteworthy that the SA1199 strain is a NorA efflux pump carrier with basal expression, and the observed MIC reduction in this strain may indicate inhibition of basal NorA pump expression, as illustrated in
Figure 1 [
59].
The SA1199B strain is widely described in the literature for its high resistance to norfloxacin and other quinolones [
16,
60]. Resistance to this class of antibiotics in
S. aureus can occur via efflux pumps or by modifications in topoisomerase, the primary target of quinolones [
61]. MIC reduction in this strain, as observed in SA1199, may be associated with inhibition of previously described resistance mechanisms. The SA1199B strain overexpresses the NorA pump, and the decrease in MIC against certain antibiotics strongly suggests inhibition of this transporter, particularly when its expression is high in the bacterial cell membrane. To rule out interference from other mechanisms, it is common practice to evaluate MIC reduction in ethidium bromide, a characteristic substrate of NorA, alongside the effect of CCCP as a reference efflux pump inhibitor. Thus, the results presented in
Figure 2 suggest that CMA4DMA has potential as a NorA pump inhibitor.
Studies with natural chalcones isolated from
Arrabidaea brachypoda [
62] and synthetic chalcones [
63] have shown that, similarly to CMA4DMA, chalcones exhibit no direct antibacterial activity but significantly reduce the MIC of norfloxacin in SA1199B strains and of ethidium bromide, indicating NorA pump inhibition. Docking experiments confirmed ligand overlap with the norfloxacin binding site in NorA. These results are particularly similar to those observed with CMA4DMA, where the amino group on the phenolic ring contributed positively to MIC reduction in NorA-harboring strains [
62,
63].
In addition to the studies mentioned, the same chalcone used in the present work was investigated against
S. aureus strains K4100, carrying QacC and β-lactamases [
64], carrying MepA [
65]. No intrinsic activity (MIC ≥ 1024 µg/mL) was observed in either study. In the K4100 strain, CMA4DMA did not inhibit β-lactamase but significantly reduced the MIC of EtBr from 64 to 32 µg/mL. In the previously mentioned study against strain K2068, significant MIC reductions for ciprofloxacin and ethidium bromide were observed. Docking studies confirmed that the chalcone can bind to the same site occupied by the standard chlorpromazine inhibitor, interacting with key protein residues, which supports its potential as a MepA inhibitor, as it may in [
65].
These studies complement and validate the results obtained in the present work, demonstrating that CMA4DMA consistently reduces efflux-mediated resistance in different S. aureus genetic backgrounds (NorA, MepA, and QacC), consolidating it as a promising antibacterial adjuvant candidate.
According to
Figure 3, the increase in fluorescence due to greater intracellular ethidium bromide accumulation corroborates the MIC reduction results, supporting efflux pump inhibition. CMA4DMA was able to promote increased intracellular EtBr, making it more available to intercalate with bacterial DNA as a consequence of efflux pump inhibition, which normally extrudes the compound from the cell [
66].
Studies of intracellular bromide accumulation using CMA4DMA in
S. aureus 1199B are nonexistent, but there are studies with other chalcones that show inhibition of the efflux pump with increased fluorescence due to ethidium bromide accumulation, as in the study by [
67], which evaluated a library of 117 chalcones for inhibitory activity of the NorA pump mediated by EtBr efflux. Furthermore, the study by [
68] evaluated the action of chalcone derivatives capable of increasing EtBr accumulation in
S. aureus by inhibiting efflux pumps. Therefore, these two studies corroborate the present study by demonstrating the ability of chalcones to increase the amount of intracellular bromide, related to efflux protein inhibition.
Therefore, according to
Figure 4, the chalcone CMA4DMA did not alter membrane permeability, and thus its effect cannot be attributed to indirect efflux pump inhibition via changes in membrane fluidity. Although indirect inhibition through membrane alteration is one mode of action for compounds targeting efflux pumps, direct interaction with the pump is more common [
69,
70]. Exceptions exist, such as in the study by [
71], where menadione was shown to act both directly on the efflux pump and indirectly on the plasma membrane, the latter also potentially disabling the pump. It is likely that CMA4DMA acts at a binding site on the NorA protein, as suggested by molecular docking studies.
The docking results indicate that CMA4DMA can effectively bind to the same NorA efflux pump domain where fluoroquinolone antibiotics such as Norfloxacin and Ciprofloxacin interact. The more favorable binding affinity of CMA4DMA compared to Norfloxacin suggests a higher energetic stability of this interaction, reinforcing its potential as a modulator of efflux activity. The affinity energy of CMA4DMA is higher (i.e., has a more negative value) when compared to natural compounds that bind to NorA and have the potential to inhibit strain 1199B. These potential increases as values decrease from <–5.0 kcal/mol [
45,
72].
The hydrophobic interactions of CMA4DMA with aliphatic residues (Leu218, Ile309, Arg310, and Ile313), distinct from the predominantly aromatic interactions observed for Norfloxacin and EtBr, reveal that CMA4DMA occupies the cavity in a differentiated binding mode. This divergence may favor synergistic effects with antibiotics, as the compound could alter the conformational dynamics of the binding pocket. This phenomenon has also been observed in molecular docking studies associated with strain 1199B inhibition [
45], where NorA ligand candidates can act synergistically with other known substrates, such as Norfloxacin.
Molecular recognition was applied in the analysis of molecular docking simulations, where it was possible to observe that the hydrophobic nature of chalcone would affect the van der Waals dispersive forces in hydrophobic interactions with the protein. The results demonstrate the significant contribution of the aromatic rings of the ligand in establishing hydrophobic interactions with the alkyl portion of the residues Leu218, Ile309, and Ile313, thereby establishing a privileged scaffold that facilitates the selection of lead compounds that inhibit the NorA efflux pump [
48,
73].
The hydrogen bonding observed between CMA4DMA and Thr211 further strengthens the binding profile, as the shorter donor–acceptor distance indicates more stable polar interactions compared to those of Norfloxacin. Importantly, the spatial distance of CMA4DMA relative to the CCCP control corroborates experimental findings, where CCCP potentiated the activity of Norfloxacin and EtBr, suggesting a mechanistic overlap in efflux pump inhibition. The residue has been found to be associated with the binding of known NorA substrates, such as levofloxacin, within the hydrophobic cavity of the efflux pump [
74].
Taken together, these findings support the hypothesis that CMA4DMA has a potential inhibitory effect on the NorA efflux pump, which may be potentiated in the presence of antibiotics. This interaction pattern highlights CMA4DMA as a promising candidate for further studies aimed at efflux pump modulation and antibiotic resistance reversal.
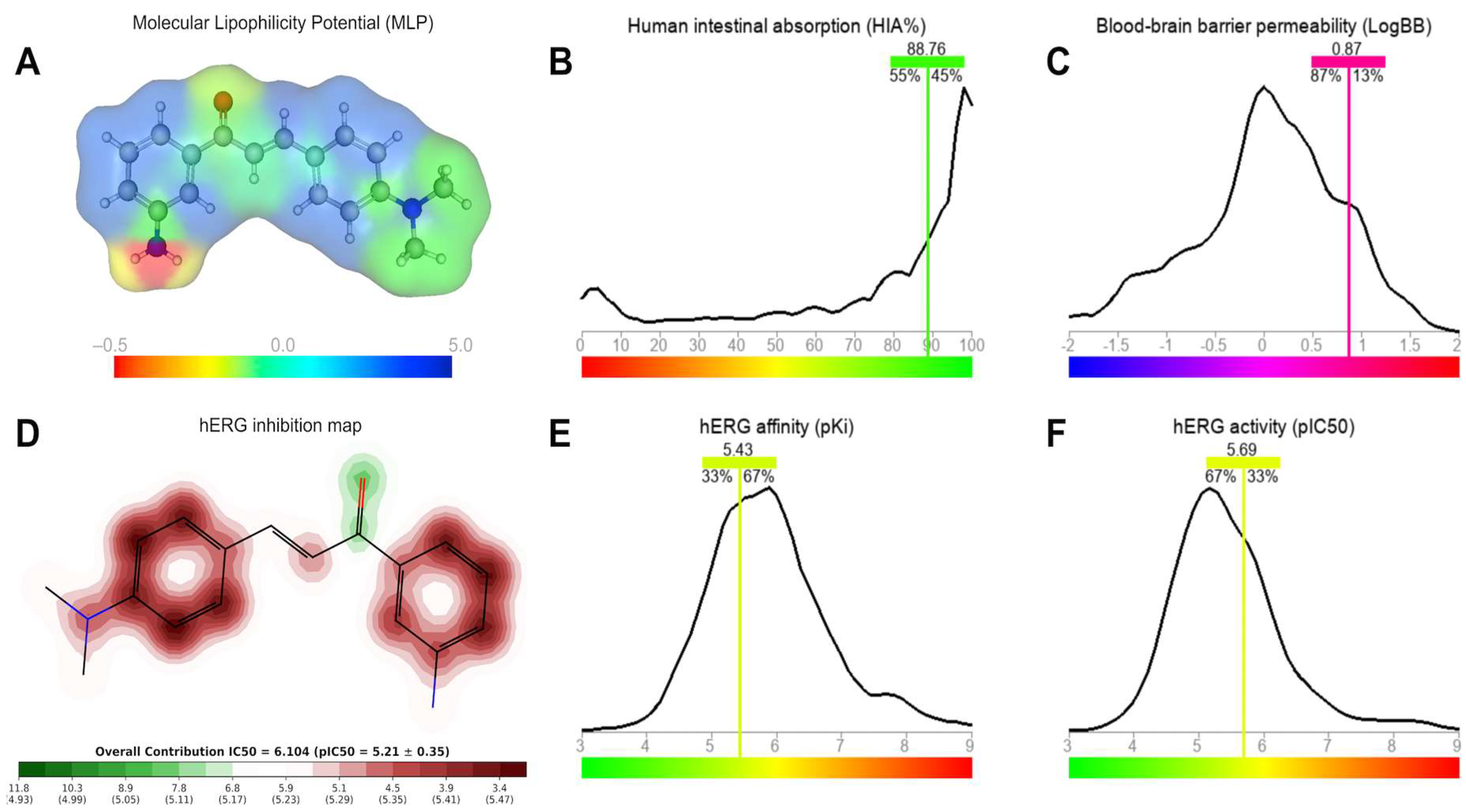
The predictive ADMET results indicate that CMA4DMA possesses physicochemical properties compatible with high cellular permeability, an attribute strongly correlated with lipophilicity (logP). Its placement in the drug-like space radar—within the thresholds of logP, MW, and TPSA—reinforces the compound’s alignment with drug-like oral properties.
The predicted Papp value in Caco-2 cells, superior to that of the reference antibiotics Norfloxacin and Ciprofloxacin, suggests that CMA4DMA may exhibit higher intestinal absorption and, consequently, improved oral bioavailability. Additionally, the prediction of high permeability in MDCK cells and the low probability of being a P-gp substrate strengthen the perspective of efficient intracellular accumulation, favoring its antimicrobial potential.
The predicted Vdss, within the acceptable range, indicates that the compound may achieve a suitable balance between plasma and tissue distribution, minimizing the risk of excessive accumulation in specific compartments. The high level of plasma protein binding (>90%), although typical of low-polarity lipophilic molecules, may influence the free fraction available to exert pharmacological activity. Nonetheless, this feature may also contribute to plasma stability and prolonged circulation time. Overall, the ADMET analysis suggests that CMA4DMA combines favorable permeability, distribution, and pharmacokinetic viability, presenting advantages over comparative antibiotics in terms of absorption and cellular transport.
The MLP map and ADMET predictions suggest that the structural balance between polar and lipophilic regions in CMA4DMA is a key determinant of its pharmacokinetic profile. The predominance of lipophilic aromatic rings combined with a single polar NH2 donor provides the compound with an optimal logP value of 2.74, which is associated with both high intestinal absorption and efficient membrane permeation. The predicted HIA of nearly 89% strongly supports the potential of CMA4DMA for oral administration, as lipophilicity is a critical determinant of absorption efficiency.
The predicted logBB and MDCK permeability values indicate that CMA4DMA may cross the BBB, displaying distribution patterns consistent with a large proportion of CNS-active compounds. While this property could support antimicrobial efficacy in tissues with selective barriers, it also raises concerns regarding potential off-target CNS effects.
A key safety consideration is the compound’s predicted interaction with hERG channels. The α,β-unsaturated aromatic system contributes to moderate hERG inhibition potential, with IC50 and pIC50 values suggesting partial blockade of K+ channels. The data similarity to known hERG inhibitors (33–67%) highlights a moderate but significant risk for cardiotoxicity, particularly in scenarios of chronic exposure. These predictions emphasize the need for further experimental validation, as inhibition of hERG channels is strongly associated with arrhythmias and other cardiac disorders. Together, these findings indicate that CMA4DMA displays a favorable absorption and distribution profile, but its potential to interact with cardiac ion channels warrants caution and targeted safety evaluation.
The metabolic prediction highlights the –N(CH3)2 group of CMA4DMA as a major liability, acting as a specific site for N-dealkylation mediated by CYP450 isoforms. While this pathway contributes to clearance, it also poses a potential safety risk due to the possible generation of reactive intermediates such as aldehydes and free radicals, which could contribute to hepatotoxicity. This aligns with the observed moderate oral LD50, which falls just below the threshold of non-toxic compounds, suggesting a risk of toxicity linked to metabolic activation.
Compared with Norfloxacin and Ciprofloxacin, CMA4DMA appears to be less metabolically stable, as reflected by its higher predicted hepatic and microsomal clearance values. This reduced metabolic stability implies lower oral bioavailability, as the compound would be more extensively metabolized during first-pass hepatic processing.
The predicted LD50 values across different administration routes emphasize the role of first-pass metabolism in mitigating systemic toxicity. While oral administration may partially protect against systemic adverse effects, bypassing hepatic metabolism through intravenous or intraperitoneal administration results in greater predicted toxicity. Interestingly, the subcutaneous route showed an intermediate LD50, suggesting potential viability for localized or topical applications, where systemic exposure could be minimized.
Taken together, these predictions suggest that although CMA4DMA has promising pharmacological attributes, its metabolic liability and associated toxicity risks require careful consideration. Optimization of structural features to reduce CYP-mediated N-dealkylation, or the exploration of alternative administration routes, may be essential for improving its therapeutic potential.