Abstract
The goal of this review is to shed light on the management of orofacial discomfort after a cancer diagnosis in the head and neck region. A search was conducted on PubMed, Scopus, and Web of Science to identify studies on postoperative pain control in oral cancer. The review included open-access research, investigations into pain management, randomized clinical trials, retrospective studies, case-control studies, prospective studies, English-written studies, and full-text publications. Exclusion criteria included animal studies; in vitro studies; off-topic studies; reviews, case reports, letters, or comments; and non-English language. Three reviewers independently accessed databases and assigned a quality rating to the chosen articles. The review explores postoperative pain management in oral cancer patients; highlighting persistent opioid use; the efficacy of adjuvant drugs, such as gabapentin; and a multimodal approach. It emphasizes the need for personalized pain management, recognizing individual pain perception and tailoring interventions. Integrating pharmacological and non-pharmacological strategies is crucial for comprehensive pain management. The review also serves as a guide for future research, emphasizing the need for standardized methodologies and diverse participant populations.
1. Introduction
Pain is characterized as “a disagreeable sensory or emotional encounter linked with real or potential damage to tissue, or articulated about such damage” [1,2]. The severity of pain frequently does not correlate with the nature or degree of tissue injury [3]. The perception of pain is individual and is a multifaceted phenomenon that encompasses psychological and emotional processes along with nociceptive and non-nociceptive impulses in ascending pathways [4,5,6]. These pathways are, in turn, connected to the activation of descending pain-inhibitory systems [2,7,8].
Cancer-related pain may arise from tumor progression, invasive diagnostic or therapeutic procedures, chemotherapy and radiotherapy toxicity, infections, or muscular pains [9,10,11,12,13]. The general belief is that cancer pain can be effectively managed in around 90 out of 100 patients [14,15]. Despite this, undertreatment is prevalent, likely stemming from factors such as negative attitudes towards the use of certain pain relief drugs and a lack of knowledge among physicians regarding effective management [16,17,18,19,20,21,22].
Numerous challenges contribute to the complexity of managing pain in head and neck cancer: the extensive innervation of the head and neck, the development of pain resulting from chemotherapy and radiotherapy, the invasive characteristics of tumors in this area, and the occurrence of pain triggered by functional movements (such as chewing, swallowing, or talking) [23,24,25,26,27,28,29].
1.1. Prevalence
In cases of oral cavity tumors, pain may be the symptom that precedes diagnosis. In fact, in this regard, more than 60% of patients with cancer in the head and neck region report discomfort or actual pain in the first 6 months [30,31,32,33,34,35,36,37].
In patients with oral squamous cell carcinoma (SCC), the most often reported symptom is oral discomfort and, later, also pain [38,39,40,41,42]. The latter results frequently occur in the anterior portion of the tongue, while lesions located at the base of the tongue mainly cause odynophagia, otalgia, and dysphagia [43,44,45,46]. These symptoms have been recorded with a higher prevalence in men than in women [47,48,49,50,51,52,53].
1.2. Management of Oral Cancer Pain
The World Health Organization (WHO) has advocated for the utilization of a rigorously validated methodology, referred to as the WHO scale, to address pain according to its intensity [54,55]. This protocol is grounded in five fundamental principles for the pharmacological management of cancer-related pain: by the mouth, by the clock, by the ladder, for the individual, and with attention to detail [56,57].
The initial approach involves the utilization of non-steroidal anti-inflammatory drugs (NSAIDs) e.g., naproxen, diclofenac, or indomethacin, for managing mild-to-moderate pain [58,59]. If the pain persists, an opioid (such as codeine or hydrocodone) should be introduced in conjunction with the NSAID [60,61,62,63,64,65]. For persistent or initially moderate-to-severe pain, an escalation in opioid potency (mainly morphine, methadone, or fentanyl) is recommended [66,67,68]. In cases where oral administration is not feasible, an exploration of rectal or transdermal routes is advised, reserving parenteral routes (such as subcutaneous or intravenous) for situations where simpler methods prove ineffective [69,70,71].
Surgical interventions prove beneficial for specific patients by reducing tumor size through debulking, consequently alleviating symptoms associated with obstruction or compression [72,73,74]. Pain management typically takes a secondary role during curative tumor resection, while it commonly becomes the primary objective in palliative surgery for unresectable tumors (Figure 1) [75,76,77].
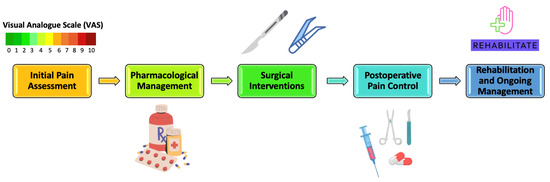
Figure 1.
Pain Management Spectrum for Oral Cancer Patients.
1.3. Management of Oral Cancer Pain after Therapy
Surgical interventions can result in substantial alterations in anatomy and functionality for patients, necessitating subsequent rehabilitation and ongoing pain management [78,79]. Employing precise surgical techniques can mitigate the severity of postoperative pain [80,81]. The careful handling of tissues, utilization of nerve- and vessel-sparing procedures, avoidance of tissue ischemia, and selection of non-muscle-splitting incisions collectively contribute to minimizing surgical pain and facilitating recovery [82,83]. Immediately after the operation, a comprehensive pain-control approach may be applied [84,85].
While numerous adverse effects of cancer treatment are now well managed, certain outcomes, such as mucositis and salivary gland hypofunction, remain nearly inevitable consequences of oral cancer treatment [86,87,88]. Mucositis, particularly during chemotherapy and radiation, is a debilitating and painful condition with potential interruptions and dose reductions impacting treatment outcomes [81,89,90,91,92,93,94,95]. Pain intensity in mucositis is linked to tissue damage and local inflammation, requiring aggressive analgesic management, often involving opioids [96]. There is no conclusive evidence favoring patient-controlled analgesia over continuous infusion, but the former results in reduced opioid usage per hour and shorter pain duration [97,98].
Laser treatment is also thought to be beneficial in avoiding and relieving mouth discomfort caused by radiation therapy and chemotherapy [99,100,101].
Although the literature on the oral cancer’s management is scarce even today, the purpose of this review is precisely to shed light on the management of orofacial pain following cancer onset in the head and neck region.
2. Materials and Methods
2.1. Protocol and Registration
The protocol for this systematic review was registered at PROSPERO with the ID: CRD 501771, and it was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [102].
2.2. Search Processing
To identify studies on the subject of postoperative pain control in oral cancer, a search was conducted on PubMed, Scopus, and Web of Science for publications published between 1 January 2003 and 31 December 2023. Boolean keywords were employed in the search strategy: (oral cancer) AND ((postoperative pain) OR (postoperative analgesia)) (Table 1).

Table 1.
Database search indicators.
2.3. Inclusion Criteria
The subsequent inclusion criteria were taken into consideration: (1) research with open access; (2) investigations into the management of pain following surgery in cases of oral cancer; (3) randomized clinical trials, retrospective studies, case-control studies, and prospective studies; (4) studies written in English; and (5) full-text publications.
Papers that did not meet the specified requirements were not accepted.
The review was conducted using the PICOS criteria:
- Participants: adults, both male and female;
- Interventions: pain control in the oral cancer;
- Comparisons: different drugs utilized;
- Outcomes: the review underscores diverse drug interventions in managing oral cancer pain, emphasizing the need for nuanced, patient-specific approaches and calling for more in-depth research in the field.
- Study: randomized clinical trials, retrospective studies, case-control studies, and prospective studies.
2.4. Exclusion Criteria
The exclusion criteria were as follows: (1) animal studies; (2) in vitro studies; (3) off-topic; (4) reviews, case reports, case series, letters, or comments; (5) non-English language.
2.5. Data Processing
Based on selection criteria, three reviewers (M.G., I.P., and R.M.) independently accessed the databases to gather the studies and assigned a quality rating. Zotero (version 6.0.15) was loaded with the chosen articles. Disagreements amongst the three writers were resolved through consultation with a senior reviewer (F.I.).
3. Results
Study Selection and Characteristics
A total of 2933 publications were found using the electronic database search (Scopus n = 1656, PubMed n = 656, Web of Science n = 621), but no articles were found using the manual search.
Following the removal of duplicates (n = 710), the titles and abstracts of 2223 studies were assessed in order to filter them. An amount of 97 records were chosen out of 2126 papers that did not match the inclusion criteria (1881 off-topic, 115 reviews, 107 vitro experiments, 23 animal research). Six records that could not be retrieved were subsequently eliminated, and ninety-one reports that did not fit the inclusion requirements were eliminated as well (seventy-four off-topic, six reviews). Eleven records were chosen for a qualitative analysis after being deemed eligible. Figure 2 and Table 2, respectively, present the selection procedure and the summary of the records that were chosen.
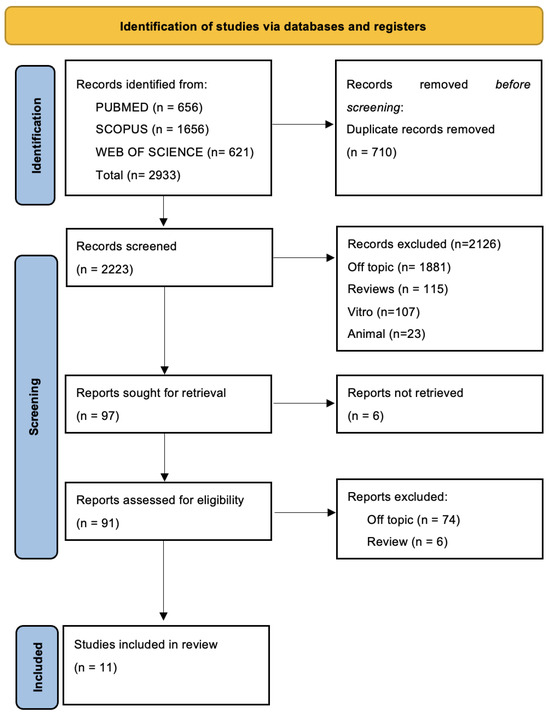
Figure 2.
PRISMA flow diagram and database search indicators.

Table 2.
Descriptive summary of item selection.
4. Discussion
Managing pain in individuals with oral cancer poses a significant challenge for healthcare practitioners, necessitating a thorough and individualized approach. The scientific literature offers a comprehensive and varied overview of methods, tactics, and treatments aimed at enhancing postoperative pain management and enhancing the overall well-being of patients undergoing surgical procedures. A review of the literature allows for an in-depth exploration of different perspectives presented in research studies, investigating crucial aspects that define the approach to pain control in patients with oral cancer.
4.1. Persistent Opioid Use and Pre-Operative Factors
A critical point underscored in the literature is the continued use of opioids during the postoperative phase. Cata et al.’s study, focusing on pain management in patients undergoing surgery for oral tongue tumors from January 2004 to January 2018, reveals that 15% of patients continued to use opioids one year after the surgery. This underscores the importance of implementing perioperative measures to effectively address pain in this specific patient cohort. Furthermore, Cata et al.’s study identified significant correlations between adjuvant therapies, preoperative opioid use, and preoperative pain with chronic and persistent postoperative opioid use [103]. In contrast to other malignancies, patients with oral tongue cancer demonstrated a higher likelihood of chronic postoperative opioid use, emphasizing the need to consider variables such as pain severity and opioid use before surgery when predicting long-term opioid use.
Another intriguing approach emerging from the literature is the use of dexmedetomidine in postoperative pain management. Gupta et al.’s study compared the efficacy of dexmedetomidine and fentanyl in oral cancer surgery patients [107]. The results showed better hemodynamic stability, reduced postoperative pain, and lower analgesic requirements in the dexmedetomidine group compared to the fentanyl group. Although both groups exhibited increased IL-6 and CRP levels postoperatively, without significant differences, further research is needed to validate the efficacy of dexmedetomidine in oral cancer pain management.
A multimodal approach to pain management was explored in Gunjan et al.’s study, involving three groups of patients undergoing oral cancer surgeries [108]. The multimodal approach in Group C, combining dexmedetomidine with nerve blocks and fentanyl, demonstrated superior pain control, reduced analgesic requirements, and higher patient satisfaction compared to other groups. The study highlights the potential benefits of this comprehensive pain management strategy for oral cancer.
Nair et al. investigated pain control in patients with head and neck cancers undergoing surgery [112]. The use of dexmedetomidine effectively attenuated the stress response, reduced bleeding, ensured smooth emergence, and enhanced tube tolerance in head and neck oncosurgeries. The findings suggested that Dexmed could be a valuable adjuvant in the management of postsurgical patients, offering stable hemodynamics and improved outcomes in major head and neck surgeries.
4.2. Efficacy of Adjuvant Therapies
Another interesting approach emerging from the literature is the use of gabapentin as an adjuvant in postoperative pain management. Chiu et al.’s study involved fifty patients divided into two groups, one treated with gabapentin and the other a control group. The preoperative administration of gabapentin demonstrated a significant reduction in postoperative pain, measured through decreased VAS scores and reduced morphine usage in the first 24 h post-surgery [104]. This suggests that gabapentin might have opioid-sparing benefits, reducing the need for morphine immediately after surgical procedures.
However, it is essential to note that Chiu et al.’s study acknowledges some limitations, such as its retrospective design, highlighting the need for additional research to establish the ideal dosage, cost-effectiveness, and long-term impacts on patient outcomes [104]. Despite these limitations, gabapentin emerges as a valuable adjuvant in oral cancer surgery.
In the context of postoperative pain management, the use of NSAIDs plays a relevant role. Puttaswamy et al.’s study compared aceclofenac and diclofenac in postoperative pain management for patients undergoing composite resection for oral cancer [106]. Both drugs effectively managed pain, with FLACC scores below three and a large decrease in VAS scores at 72 h post-surgery. The study indicates that aceclofenac could be considered a suitable alternative for pain management in oral cancer patients, particularly for those with a history of gastritis or peptic ulcers.
Another significant contribution to the literature comes from the research conducted by Yaguchi et al., focusing on the use of hydroxyethyl starch in oral cancer surgeries [105]. The study demonstrated that the administration of hydroxyethyl starch effectively maintained circulation without increasing intraoperative blood loss or adversely affecting renal function, even at lower doses. While acknowledging limitations, such as its retrospective nature and short follow-up period, the study suggests the potential effectiveness and safety of hydroxyethyl starch in oral cancer surgeries, providing valuable insights for further exploration.
4.3. Multimodal Approaches
Amiri et al.’s research delved into the effectiveness of preemptive analgesia (PAND) in radical neck dissection surgery for oral cancer patients [109]. The PAND regimen, comprising pregabalin, acetaminophen, naproxen, and dextromethorphan, significantly reduced postoperative pain and opioid analgesic requirements compared to the control group. The study suggested the need for further research to refine preemptive analgesic protocols for improved pain management in radical neck dissection surgery.
Another significant contribution comes from Singhal et al.’s investigation into postoperative pain control in oral cancer patients undergoing a commando operation with PMMF reconstruction [110]. The use of epidural morphine showed superior analgesia compared to intravenous morphine in oral cancer surgery with flap reconstruction. The study suggested that epidural morphine offers enhanced postoperative pain management, emphasizing its potential benefits over intravenous morphine.
Sjamsudin et al. aimed to investigate pain control in patients with stage 3 and 4 oral squamous cell carcinoma (OSCC) undergoing surgery as the primary treatment [113]. The study revealed a significant decrease in postoperative pain and anxiety and an increase in quality of life. The findings suggest the effectiveness of operative therapy in reducing pain and anxiety levels while enhancing the overall quality of life in OSCC patients.
4.4. Holistic Approaches
Jiang et al. analyzed postoperative oral cancer cases, comparing observation and control groups [111]. The study indicated significant differences in oral care effects, tumor marker expression, immune capacity, and patient satisfaction between the two groups. The combination of traditional Chinese medicine (TCM) anticancer decoction, chemotherapy, and nursing intervention demonstrated enhanced clinical efficacy, reduced adverse reactions, and improved patient outcomes. The study suggests that this comprehensive approach positively influences immune function, patient satisfaction, and survival quality in postoperative oral cancer cases.
In conclusion, the scientific literature thoroughly examines various perspectives and approaches to pain management in patients with oral cancer undergoing surgical interventions. From persistent opioid use to the assessment of adjuvant drugs, such as gabapentin, from multimodal approaches to preventive therapies, each study contributes to outlining a comprehensive framework of strategies aimed at improving patients’ quality of life and reducing postoperative pain. A personalized approach and the integration of different therapeutic modalities emerge as key elements in postoperative pain management in patients with oral cancer, offering valuable insights for clinical practice and guiding the direction of future studies in this field.
In recent years, the integration of genomics and precision medicine has revolutionized the landscape of oral cancer treatment. By elucidating the genetic alterations and molecular pathways underlying the development and progression of oral cancers, genomics has enabled the identification of novel therapeutic targets and personalized treatment strategies [114]. Precision medicine approaches, leveraging genomic profiling and molecular diagnostics, empower clinicians to tailor treatment regimens to the unique genetic profile of individual patients. This paradigm shift from a one-size-fits-all approach to a patient-centric model holds immense promise in optimizing treatment outcomes and minimizing adverse effects in oral cancer patients. Furthermore, the ongoing advancements in high-throughput sequencing technologies and computational algorithms continue to propel the field forward, fostering a deeper understanding of the molecular complexities of oral cancer and paving the way for innovative targeted therapies and precision oncology interventions [5,40].
5. Limitations
The review on postoperative pain control in oral cancer patients, while contributing valuable insights, is not without limitations that warrant careful consideration. One prominent constraint is the limited availability of studies addressing the specific nuances of managing pain in oral cancer cases. This scarcity, while not uncommon in emerging fields, underscores the need for further extensive research to establish a more robust knowledge base.
Another potential limitation is the inclusion criteria that exclusively favored English-language studies. This decision introduces a language bias, potentially excluding pertinent research conducted in languages other than English. The consequence is a potential oversight of valuable contributions from non-English literature, limiting the depth and inclusivity of the review.
The studies analyzed exhibit significant heterogeneity, as reflected in their diverse methodologies and sample sizes. Specifically, a subset of studies adopts a retrospective design with relatively small sample sizes, potentially limiting the generalizability and robustness of the findings. This heterogeneity introduces challenges in synthesizing results cohesively and may impede the ability to draw definitive conclusions regarding postoperative pain management in oral cancer patients. Consequently, caution is warranted when interpreting the outcomes, and further research incorporating larger, prospective studies is needed to validate and expand upon the findings obtained from these varied study designs.
The diversity in methodologies and quality assessments across these studies poses a challenge in synthesizing findings cohesively, potentially impacting the overall strength of the evidence presented.
Publication bias, a phenomenon where journals tend to favor publishing studies with positive or statistically significant results, may influence the review’s conclusions. The exclusion of studies with negative or inconclusive findings may skew the overall interpretation of the literature towards more optimistic outcomes.
While the search strategy utilizing Boolean keywords is a strength, the possibility of missing relevant studies cannot be entirely ruled out. Variations in terminologies or indexing across databases might lead to inadvertent omissions, affecting the comprehensiveness of the search.
Despite the effort to mitigate bias through a three-reviewer selection process, there remains a potential for subjective judgment, introducing selection bias. Differences in interpretation among reviewers could impact the inclusivity and representativeness of the studies included in the review.
6. Conclusions
The review, despite these limitations, offers a thorough exploration of diverse approaches to postoperative pain management in oral cancer patients. It sheds light on persistent opioid use; explores the efficacy of adjuvant drugs, such as gabapentin; and advocates for a multimodal approach, emphasizing the multifaceted nature of pain control. A key takeaway from the review is the critical need for a personalized approach to pain management in oral cancer patients. Recognizing the individuality of pain perception and tailoring interventions accordingly emerges as a central theme, providing clinicians with valuable insights into optimizing patient care. The integration of different therapeutic modalities, encompassing both pharmacological and non-pharmacological strategies, emerges as a crucial element in achieving comprehensive postoperative pain management. This holistic approach aligns with the evolving paradigm in healthcare that acknowledges the complexity of pain experiences and addresses them through diverse means. While offering practical insights for clinical practice, the review also acts as a guiding compass for future research. The identified limitations underscore the necessity for more extensive, rigorous studies with standardized methodologies and diverse participant populations. This imperative push for further research aims to deepen our understanding of oral cancer pain management and refine evidence-based practices in the field.
Author Contributions
Conceptualization, F.I., I.P., A.M., R.M. and A.M.I.; methodology, A.D.I., I.P. and M.G.; software, I.P., M.G., R.M. and G.D.; validation, F.I., A.D.I. and G.D.; formal analysis, A.D.I., I.P. and M.G.; resources, A.D.I., A.M.I. and I.P.; data curation, I.P., A.M., M.G. and G.D.; writing—original draft preparation, A.D.I., A.M.I., M.G., F.I. and R.M.; writing—review and editing, I.P., R.M. and A.M.I.; visualization, F.I., I.P., M.G., A.M. and A.M.I.; supervision, A.D.I., A.M.I., A.M., R.M., F.I. and G.D.; project administration, M.G., I.P., A.M., F.I. and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| EORTC QOL | European Organization for Research and Treatment of Cancer Quality of Life Questionnaires |
| FLACC | Face, Legs, Activity, Cry, Consolability |
| HADS | Hospital Anxiety and Depression Scale |
| NSAID | Non-steroidal anti-inflammatory drug |
| UPAT | Universal Pain Assessment Tool |
| OSCC | Oral squamous cell carcinoma |
| UCSF-OCPQ | University of California San Francisco Oral Cancer Pain Questionnaire |
| PAND | Preemptive analgesia |
| PCA | Patient-controlled analgesia |
| PMMF | Pectoralis major myocutaneous flap |
| TCM | Traditional Chinese Medicine |
| VAS | Visual analog scale |
References
- Treede, R.-D. The International Association for the Study of Pain Definition of Pain: As Valid in 2018 as in 1979, but in Need of Regularly Updated Footnotes. PAIN Rep. 2018, 3, e643. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L. Pain Processing in the Human Nervous System: A Selective Review of Nociceptive and Biobehavioral Pathways. Prim. Care 2012, 39, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Malcangi, G.; Patano, A.; Guglielmo, M.; Sardano, R.; Palmieri, G.; Pede, C.; Ruvo, E.; Inchingolo, A.; Mancini, A.; Inchingolo, F.; et al. Precision Medicine in Oral Health and Diseases: A Systematic Review. J. Pers. Med. 2023, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Viapiano, F.; Netti, A.; Patano, A.; Isacco, C.G.; Inchingolo, A.D.; Inchingolo, F. Sixty-Month Follow Up of Clinical MRONJ Cases Treated with CGF and Piezosurgery. Bioengineering 2023, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wu, F.; Gan, X.; Yang, X.; Zhou, L.; Chen, J.; He, Y.; Zhang, R.; Zhu, B.; Liu, L. The Involvement of Descending Pain Inhibitory System in Electroacupuncture-Induced Analgesia. Front. Integr. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.M. The Treatment of Cancer Pain. N. Engl. J. Med. 1985, 313, 84–95. [Google Scholar] [CrossRef]
- Cramer, J.D.; Johnson, J.T.; Nilsen, M.L. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of-Life Impact. Otolaryngol. Head Neck Surg. 2018, 159, 853–858. [Google Scholar] [CrossRef]
- Burton, A.W.; Fanciullo, G.J.; Beasley, R.D.; Fisch, M.J. Chronic Pain in the Cancer Survivor: A New Frontier. Pain Med. 2007, 8, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Santacroce, L.; Ballini, A.; Topi, S.; Dipalma, G.; Haxhirexha, K.; Bottalico, L.; Charitos, I.A. Oral Cancer: A Historical Review. Int. J. Environ. Res. Public Health 2020, 17, 3168. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Ballini, A.; Georgakopoulos, P.G.; Inchingolo, A.M.; Tsantis, S.; De Vito, D.; Cantore, S.; Georgakopoulos, I.P.; Dipalma, G. Immediate Implant Placement by Using Bone-Albumin Allograft and Concentrated Growth Factors (CGFS): Preliminary Results of a Pilot Study. Oral Implantol. 2018, 11, 47–56. [Google Scholar]
- Kumar, S.P. Cancer Pain: A Critical Review of Mechanism-Based Classification and Physical Therapy Management in Palliative Care. Indian J. Palliat. Care 2011, 17, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.R. The Challenge of Cancer Pain Assessment. Ulst. Med. J. 2021, 90, 37–40. [Google Scholar]
- Stjernswärd, J. WHO Cancer Pain Relief Programme. Cancer Surv. 1988, 7, 195–208. [Google Scholar] [PubMed]
- Malcangi, G.; Patano, A.; Ciocia, A.M.; Netti, A.; Viapiano, F.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Inchingolo, A.D.; Dipalma, G.; et al. Benefits of Natural Antioxidants on Oral Health. Antioxidants 2023, 12, 1309. [Google Scholar] [CrossRef]
- Mercadante, S.; Prestia, G.; Ranieri, M.; Giarratano, A.; Casuccio, A. Opioid Use and Effectiveness of Its Prescription at Discharge in an Acute Pain Relief and Palliative Care Unit. Support. Care Cancer 2013, 21, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.D.; Eguchi, M.; Stokes, W.A.; Amini, A.; Hararah, M.; Ding, D.; Valentine, A.; Bradley, C.J.; Karam, S.D. Short- and Long-Term Opioid Use in Patients with Oral and Oropharynx Cancer. Otolaryngol. Head Neck Surg. 2019, 160, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, C.A.; Roberts, L.J.; Somogyi, A.A.; MacIntyre, P.E. Acute Pain Management in Opioid-Tolerant Patients: A Growing Challenge. Anaesth. Intensive Care 2011, 39, 804–823. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A.; Portenoy, R.; Lacchetti, C.; Campbell, T.; Cheville, A.; Citron, M.; Constine, L.S.; Cooper, A.; Glare, P.; Keefe, F.; et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3325–3345. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Ballini, A.; Mura, S.A.; Farronato, D.; Cirulli, N.; Dds, F.; Gheno, E.; Vermesan, D.; Pederzoli, P.; Resta, G.; et al. Use of Platelet Rich Fibrin and Bio-OSS/SINT-Oss for Implant-Prosthetic Rehabilitation in Maxillary Atrophy with Sinus Pathology: A 48-Month Follow-Up. Eur. J. Inflamm. 2015, 13, 58–65. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Servili, A.; Inchingolo, A.M.; Dipalma, G. A Hypothetical Correlation between Hyaluronic Acid Gel and Development of Cutaneous Metaplastic Synovial Cyst. Head Face Med. 2010, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another Look at Dietary Polyphenols: Challenges in Cancer Preventionand Treatment. Curr. Med. Chem. 2022, 29, 1061–1082. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Pede, C.D.; et al. The Efficacy of a New AMCOP® Elastodontic Protocol for Orthodontic Interceptive Treatment: A Case Series and Literature Overview. Int. J. Environ. Res. Public Health 2022, 19, 988. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Villabruna, B.; Inchingolo, A.M.; Dipalma, G. Severe Anisocoria after Oral Surgery under General Anesthesia. Int. J. Med. Sci. 2010, 7, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Inchingolo, A.D.; Lazzaro, R.; Aityan, S.K.; Maggiore, M.E.; Mancini, A.; Laforgia, R.; et al. Sars-CoV-2 Virus Infection May Interfere CD34+ Hematopoietic Stem Cells and Megakaryocyte–Erythroid Progenitors Differentiation Contributing to Platelet Defection towards Insurgence of Thrombocytopenia and Thrombophilia. Microorganisms 2021, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Santacroce, L.; Cantore, S.; Ballini, A.; Del Prete, R.; Topi, S.; Saini, R.; Dipalma, G.; Arrigoni, R. Probiotics and EpiCor® in Human Health. J. Biol. Regul. Homeost. Agents 2019, 33, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Mahanna, G.K. Cancer in the Differential Diagnosis of Orofacial Pain. Dent. Clin. N. Am. 1997, 41, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Emerton, S.; Kolbinson, D.A.; Le, N.D.; Phillips, N.; Stevenson-Moore, P.; Osoba, D. Quality of Life and Oral Function Following Radiotherapy for Head and Neck Cancer. Head Neck 1999, 21, 1–11. [Google Scholar] [CrossRef]
- Jensen, O.M. International Variation in the Incidence of Cancer of the Upper Digestive and Respiratory Tract. Adv. Otorhinolaryngol. 1991, 46, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Hart-Johnson, T.; Loeffler, D.R. Cancer-Related Chronic Pain: Examining Quality of Life in Diverse Cancer Survivors. Cancer 2011, 117, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Maldotti, F.; Crema, L.; Malagò, M.; Ciorba, A. Pain in Head and Neck Cancer: Prevalence and Possible Predictive Factors. J. BUON 2014, 19, 592–597. [Google Scholar] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-Syndromic Multiple Supernumerary Teeth in a Family Unit with a Normal Karyotype: Case Report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Chaturvedi, S.; Abdullah, S.; Rajput, G.; Alqahtani, N.M.; Chaturvedi, M.; Gurumurthy, V.; Saini, R.; Bavabeedu, S.S.; Minervini, G. Clinical Trial to Assess Physiology and Activity of Masticatory Muscles of Complete Denture Wearer Following Vitamin D Intervention. Medicina 2023, 59, 410. [Google Scholar] [CrossRef]
- Reddy, L.K.V.; Madithati, P.; Narapureddy, B.R.; Ravula, S.R.; Vaddamanu, S.K.; Alhamoudi, F.H.; Minervini, G.; Chaturvedi, S. Perception about Health Applications (Apps) in Smartphones towards Telemedicine during COVID-19: A Cross-Sectional Study. J. Pers. Med. 2022, 12, 1920. [Google Scholar] [CrossRef]
- Epstein, J.B.; Stewart, K.H. Radiation Therapy and Pain in Patients with Head and Neck Cancer. Eur. J. Cancer B Oral Oncol. 1993, 29B, 191–199. [Google Scholar] [CrossRef]
- Cuffari, L.; Tesseroli de Siqueira, J.T.; Nemr, K.; Rapaport, A. Pain Complaint as the First Symptom of Oral Cancer: A Descriptive Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Malcangi, G.; Piras, F.; Palmieri, G.; Settanni, V.; Riccaldo, L.; Morolla, R.; Buongiorno, S.; de Ruvo, E.; Inchingolo, A.D.; et al. Precision Medicine on the Effects of Microbiota on Head-Neck Diseases and Biomarkers Diagnosis. J. Pers. Med. 2023, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, R.; Scolaro, A.; Boccalari, E.; Dolci, C.; Scarano, A.; Inchingolo, F.; Ravazzani, P.; Muti, P.; Tartaglia, G. Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers. Biosensors 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Corelli, R.; Inchingolo, A.M.; Dipalma, G. Upper Eyelid Reconstruction: A Short Report of an Eyelid Defect Following a Thermal Burn. Head Face Med. 2009, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Hechler, B.; Carlson, E.R.; Heidel, R.E.; Fahmy, M.D.; McCoy, J.M. Are Oral Pain and Otalgia Predictive of Perineural Invasion in Squamous Cell Carcinoma of the Oral Tongue? J. Oral Maxillofac. Surg. 2020, 78, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Haidari, S.; Boser, S.; Troeltzsch, M.; Probst, F.A.; Ehrenfeld, M.; Otto, S. What Factors Are Associated with Regional Recurrence After Operative Treatment of Oral Squamous Cell Carcinoma? J. Oral Maxillofac. Surg. 2018, 76, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Scarbrough, T.J.; Day, T.A.; Williams, T.E.; Hardin, J.H.; Aguero, E.G.; Thomas, C.R. Referred Otalgia in Head and Neck Cancer: A Unifying Schema. Am. J. Clin. Oncol. 2003, 26, e157–e162. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Tarullo, A.; Inchingolo, A.D.; Dipalma, G.; Podo Brunetti, S.; Tarullo, A.; Cagiano, R. Combined Occlusal and Pharmacological Therapy in the Treatment of Temporo-Mandibular Disorders. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1296–1300. [Google Scholar] [PubMed]
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and Growth Factors in Oral Squamous Cell Carcinoma: Useful Source of Dental-Derived Stem Cells to Develop a Steroidogenic Model in New Clinical Strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral Infection by Staphylococcus aureus in Patients Affected by White Sponge Nevus: A Description of Two Cases Occurred in the Same Family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral Probiotics Influence Oral and Respiratory Tract Infections in Pediatric Population: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Okuyemi, O.T. Malignant Salivary Gland Tumors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563022/ (accessed on 12 January 2023).
- Fanali, S.; Tumedei, M.; Pignatelli, P.; Inchingolo, F.; Pennacchietti, P.; Pace, G.; Piattelli, A. Implant Primary Stability with an Osteocondensation Drilling Protocol in Different Density Polyurethane Blocks. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ventafridda, V.; Saita, L.; Ripamonti, C.; De Conno, F. WHO Guidelines for the Use of Analgesics in Cancer Pain. Int. J. Tissue React. 1985, 7, 93–96. [Google Scholar] [PubMed]
- Jadad, A.R.; Browman, G.P. The WHO Analgesic Ladder for Cancer Pain Management. Stepping up the Quality of Its Evaluation. JAMA 1995, 274, 1870–1873. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Pain Relief and Palliative Care. Report of a WHO Expert Committee; World Health Organization Technical Report Series 804; World Health Organization: Geneva, Switzerland, 1990; pp. 1–75. [Google Scholar]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Slater, D.; Kunnathil, S.; McBride, J.; Koppala, R. Pharmacology of Nonsteroidal Antiinflammatory Drugs and Opioids. Semin. Interv. Radiol. 2010, 27, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Mijiti, A.; Moming, A. Survival Pattern and Prognostic Factors of Patients with Squamous Cell Carcinoma of the Tongue: A Retrospective Analysis of 210 Cases. J. Oral Maxillofac. Surg. 2013, 71, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.-K.; Li, W.-Y.; Yang, M.-H.; Chang, S.-Y.; Chu, P.-Y.; Tsai, T.-L.; Wang, Y.-F.; Chang, P.M.-H. Treatment for T1-2 Oral Squamous Cell Carcinoma with or without Perineural Invasion: Neck Dissection and Postoperative Adjuvant Therapy. Ann. Surg. Oncol. 2012, 19, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.J.; Weymuller, E.A. Assessment of Quality of Life in Head and Neck Cancer Patients. Head Neck 1993, 15, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Terrell, J.E.; Welsh, D.E.; Bradford, C.R.; Chepeha, D.B.; Esclamado, R.M.; Hogikyan, N.D.; Wolf, G.T. Pain, Quality of Life, and Spinal Accessory Nerve Status after Neck Dissection. Laryngoscope 2000, 110, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Corelli, R.; Mingrone, R.; Inchingolo, A.M.; Dipalma, G. Simple Technique for Augmentation of the Facial Soft Tissue. Sci. World J. 2012, 2012, 262989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez, R.F.; Castillo, J.M.; Del Pilar Castillo, M.; Nuñez, P.D.; Rodriguez, M.F.; Restrepo, J.M.; Rodriguez, J.M.; Ortiz, Y.; Angel, A.M. Codeine/Acetaminophen and Hydrocodone/Acetaminophen Combination Tablets for the Management of Chronic Cancer Pain in Adults: A 23-Day, Prospective, Double-Blind, Randomized, Parallel-Group Study. Clin. Ther. 2007, 29, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of Cancer Pain in Adult Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Corli, O.; Floriani, I.; Roberto, A.; Montanari, M.; Galli, F.; Greco, M.T.; Caraceni, A.; Kaasa, S.; Dragani, T.A.; Azzarello, G.; et al. Are Strong Opioids Equally Effective and Safe in the Treatment of Chronic Cancer Pain? A Multicenter Randomized Phase IV ‘Real Life’ Trial on the Variability of Response to Opioids. Ann. Oncol. 2016, 27, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Opioids, Adjuvants, and Interventional Options for Pain Management of Symptomatic Metastases—Ghosh—Annals of Palliative Medicine. Available online: https://apm.amegroups.org/article/view/4176/html (accessed on 30 January 2024).
- Samek, M.; Zech, D.; Grond, S. Pain-relieving therapy for 57 patients with malignancies in the head and neck area. Dtsch. Zahnarztl. Z. 1990, 45, 49–51. [Google Scholar] [PubMed]
- Connelly, S.T.; Schmidt, B.L. Evaluation of Pain in Patients with Oral Squamous Cell Carcinoma. J. Pain 2004, 5, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Shuman, A.G.; Terrell, J.E.; Light, E.; Wolf, G.T.; Bradford, C.R.; Chepeha, D.; Jiang, Y.; McLean, S.; Ghanem, T.A.; Duffy, S.A. Predictors of Pain among Head and Neck Cancer Patients. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.E.; Schwalk, A.J. Overview of Malignant Central Airway Obstruction. Mediastinum 2023, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Baijens, L.W.J.; Walshe, M.; Aaltonen, L.-M.; Arens, C.; Cordier, R.; Cras, P.; Crevier-Buchman, L.; Curtis, C.; Golusinski, W.; Govender, R.; et al. European White Paper: Oropharyngeal Dysphagia in Head and Neck Cancer. Eur. Arch. Otorhinolaryngol. 2021, 278, 577–616. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Zech, D.; Schug, S.A.; Lynch, J.; Lehmann, K.A. Validation of World Health Organization Guidelines for Cancer Pain Relief during the Last Days and Hours of Life. J. Pain Symptom Manag. 1991, 6, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Forbes, K. Palliative Care in Patients with Cancer of the Head and Neck. Clin. Otolaryngol. Allied Sci. 1997, 22, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.N.; Cleator, A.J.; Lowe, D.; Ghazali, N. Identifying Pain-Related Concerns in Routine Follow-up Clinics Following Oral and Oropharyngeal Cancer. World J. Clin. Oncol. 2012, 3, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Mobasheri, A.; Trovato, F.M.; Szychlinska, M.A.; Imbesi, R.; Castrogiovanni, P. Post-Operative Rehabilitation and Nutrition in Osteoarthritis. F1000Research 2016, 3, 116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Almeida, L.E.; Ronsivalle, V.; Cicciù, M. Prevalence of Temporomandibular Disorders (TMD) in Obesity Patients: A Systematic Review and Meta-analysis. J. Oral Rehabil. 2023, 50, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Ronsivalle, V.; Cicciù, M. Children Oral Health and Parents Education Status: A Cross Sectional Study. BMC Oral Health 2023, 23, 787. [Google Scholar] [CrossRef] [PubMed]
- Jacox, A.; Carr, D.B.; Payne, R. New Clinical-Practice Guidelines for the Management of Pain in Patients with Cancer. N. Engl. J. Med. 1994, 330, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Van den Beuken-van Everdingen, M. Chronic Pain in Cancer Survivors: A Growing Issue. J. Pain Palliat. Care Pharmacother. 2012, 26, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Sonis, S. Toxicities Associated with Head and Neck Cancer Treatment and Oncology-Related Clinical Trials. Curr. Probl. Cancer 2016, 40, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Garimella, V.; Cellini, C. Postoperative Pain Control. Clin. Colon Rectal Surg. 2013, 26, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M. Advances in Understanding of Toxicities of Treatment for Head and Neck Cancer. Oral Oncol. 2009, 45, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Burlage, F.R.; Spijkervet, F.K.L.; Jansma, J.; Coppes, R.P. Prevention and treatment of the consequences of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.L.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral Mucositis: The Hidden Side of Cancer Therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Isola, G.; Cicciù, M. Conservative Treatment of Temporomandibular Joint Condylar Fractures: A Systematic Review Conducted According to PRISMA Guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. J. Oral Rehabil. 2023, 50, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Post-Traumatic Stress, Prevalence of Temporomandibular Disorders in War Veterans: Systematic Review with Meta-Analysis. J. Oral Rehabil. 2023, 50, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G. The Association between Parent Education Level, Oral Health, and Oral-Related Sleep Disturbance. An Observational Crosssectional Study. Eur. J. Paediatr. Dent. 2023, 24, 218–223. [Google Scholar] [CrossRef]
- Scorzetti, L.; Marcattili, D.; Pasini, M.; Mattei, A.; Marchetti, E.; Marzo, G. Association between Obesity and Periodontal Disease in Children. Eur. J. Paediatr. Dent. 2013, 14, 181–184. [Google Scholar] [PubMed]
- Saccomanno, S.; Mummolo, S.; Giancaspro, S.; Manenti, R.J.; Mastrapasqua, R.F.; Marzo, G.; Quinzi, V. Catering Work Profession and Medico-Oral Health: A Study on 603 Subjects. Healthcare 2021, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, E.; Tecco, S.; Caterini, E.; Casalena, F.; Quinzi, V.; Mattei, A.; Marzo, G. Alcohol-Free Essential Oils Containing Mouthrinse Efficacy on Three-Day Supragingival Plaque Regrowth: A Randomized Crossover Clinical Trial. Trials 2017, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Saccomanno, S.; Quinzi, V.; D’Andrea, N.; Albani, A.; Coceani Paskay, L.; Marzo, G. Traumatic Events and Eagle Syndrome: Is There Any Correlation? A Systematic Review. Healthcare 2021, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J. Cancer Treatment-Induced Mucositis Pain: Strategies for Assessment and Management. Ther. Clin. Risk Manag. 2006, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.E.; Worthington, H.V.; Furness, S.; McCabe, M.; Khalid, T.; Meyer, S. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst. Rev. 2010, CD001973. [Google Scholar] [CrossRef] [PubMed]
- McNicol, E.D.; Ferguson, M.C.; Hudcova, J. Patient Controlled Opioid Analgesia versus Non-patient Controlled Opioid Analgesia for Postoperative Pain. Cochrane Database Syst. Rev. 2015, 2015, CD003348. [Google Scholar] [CrossRef] [PubMed]
- Buchsel, P.C. Polyvinylpyrrolidone-Sodium Hyaluronate Gel (Gelclair): A Bioadherent Oral Gel for the Treatment of Oral Mucositis and Other Painful Oral Lesions. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Zanin, T.; Zanin, F.; Carvalhosa, A.A.; de Souza Castro, P.H.; Pacheco, M.T.; Zanin, I.C.J.; Brugnera, A. Use of 660-Nm Diode Laser in the Prevention and Treatment of Human Oral Mucositis Induced by Radiotherapy and Chemotherapy. Photomed. Laser Surg. 2010, 28, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of Oral Mucositis in Patients with Cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Patino, M.; Gorur, A.; Du, K.N.; Uhelski, M.L.; Myers, J.; Lai, S.; Rubin, M.L.; Dougherty, P.M.; Owusu-Agyemang, P. Persistent and Chronic Postoperative Opioid Use in a Cohort of Patients with Oral Tongue Squamous Cell Carcinoma. Pain Med. 2019, 21, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.W.; Leung, C.C.H.; Lau, E.Y.K.; Burd, A. Analgesic Effects of Preoperative Gabapentin after Tongue Reconstruction with the Anterolateral Thigh Flap. Hong Kong Med. J. 2012, 18, 30–34. [Google Scholar] [PubMed]
- Yaguchi, E.; Ujita, T.; Hamaguchi, S. Utility of 6% Hydroxyethyl Starch 130/0.4 in Oral Cancer Surgeries with a Duration of over 6 Hours: A Retrospective Case-Control Study. Medicine 2023, 102, e32958. [Google Scholar] [CrossRef] [PubMed]
- Puttaswamy, G.; Narayana, S.; Mohiyuddin, S.A. The Efficacy and Safety of Intramuscular Aceclofenac and Diclofenac for Managing Postoperative Pain in Patients Undergoing Composite Resection for Oral Cancer. J. Cancer Metastasis Treat. 2023, 9, 7. [Google Scholar] [CrossRef]
- Gupta, M.; Kushwaha, J.K.; Singh, P.K.; Gupta, R.; Ali, W.; Verma, R.; Kapoor, R.; Nischal, A. Opioid Sparing Effect of Anaesthesia on Postoperative Inflammatory Cytokine Response in Oral Cancer Surgery. J. Clin. Diagn. Res. 2018, 12, UC10–UC15. [Google Scholar] [CrossRef]
- Gunjan; Kohli, M.; Singh, P.K.; Gupta, R.; Chaudhary, A.K.; Kumar, V.; Bogra, J. Multimodal versus Conventional Approach for Postoperative Pain Relief in Oral Cancer Patients. J. Clin. Diagn. Res. 2016, 10, UC05–UC08. [Google Scholar] [CrossRef]
- Amiri, H.R.; Mirzaei, M.; Beig Mohammadi, M.T.; Tavakoli, F. Multi-Modal Preemptive Analgesia with Pregabalin, Acetaminophen, Naproxen, and Dextromethorphan in Radical Neck Dissection Surgery: A Randomized Clinical Trial. Anesth. Pain Med. 2016, 6, e33526. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.K.; Mishra, S.; Bhatnagar, S.; Singh, R. Epidural Morphine Analgesia Compared with Intravenous Morphine for Oral Cancer Surgery with Pectoralis Major Myocutaneous Flap Reconstruction. Acta Anaesthesiol. Scand. 2006, 50, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xiao, F.; Liu, L.; Meng, Z.; Zhang, C. Effects of Traditional Chinese Medicine Anticancer Decoction Combined with Basic Chemotherapy and Nursing Intervention on Oral Cancer Patients after Surgery and Its Effect on Tumor Markers and Immune Function. BioMed Res. Int. 2022, 2022, 6341381. [Google Scholar] [CrossRef]
- Nair, V.A.; Gladston, D.V.; Krishna K. M., J.; Koshy, R.C. Effects of Intravenous Dexmedetomidine on Perioperative Haemodynamics and Quality of Emergence in Patients Undergoing Head and Neck Surgery Following General Anaesthesia—A Comparative Randomized, Double-Blind Placebo-Controlled Study. Ain-Shams J. Anesthesiol. 2022, 14, 48. [Google Scholar] [CrossRef]
- Sjamsudin, E.; Maulina, T.; Cipta, A.; Iskandarsyah, A.; Hardianto, A.; Nandini, M.; Kasim, A.; Yusuf, H.Y. Assessment of Oral Cancer Pain, Anxiety, and Quality of Life of Oral Squamous Cell Carcinoma Patients with Invasive Treatment Procedure. Oral Maxillofac. Surg. 2018, 22, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; LaMonte, S.J.; Erb, N.L.; Beckman, K.L.; Sadeghi, N.; Hutcheson, K.A.; Stubblefield, M.D.; Abbott, D.M.; Fisher, P.S.; Stein, K.D.; et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 203–239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).