Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice
Abstract
1. Introduction
2. Results
2.1. JJGW07 Showed No Affinity for 5-HT6 Receptors
2.2. JJGW07 Showed Antagonistic Properties at D2, 5-HT1A, 5-HT2A, and 5-HT7 Receptors
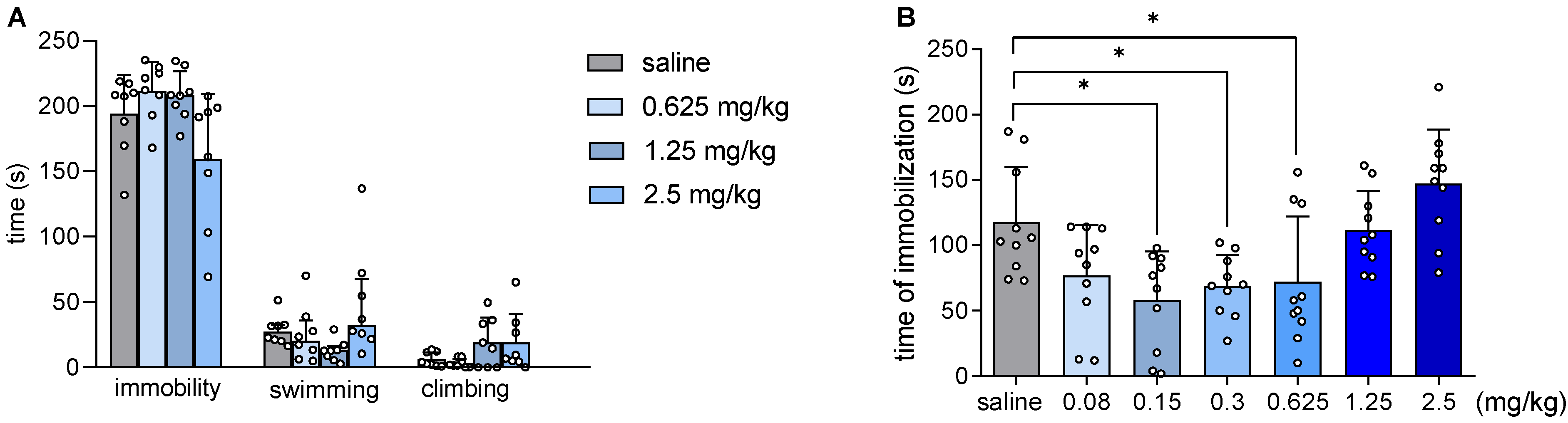
2.3. JJGW07 Did Not Affect Mouse Immobility in the Forced Swim Test and Reduced the Immobility Time in the Tail Suspension Test
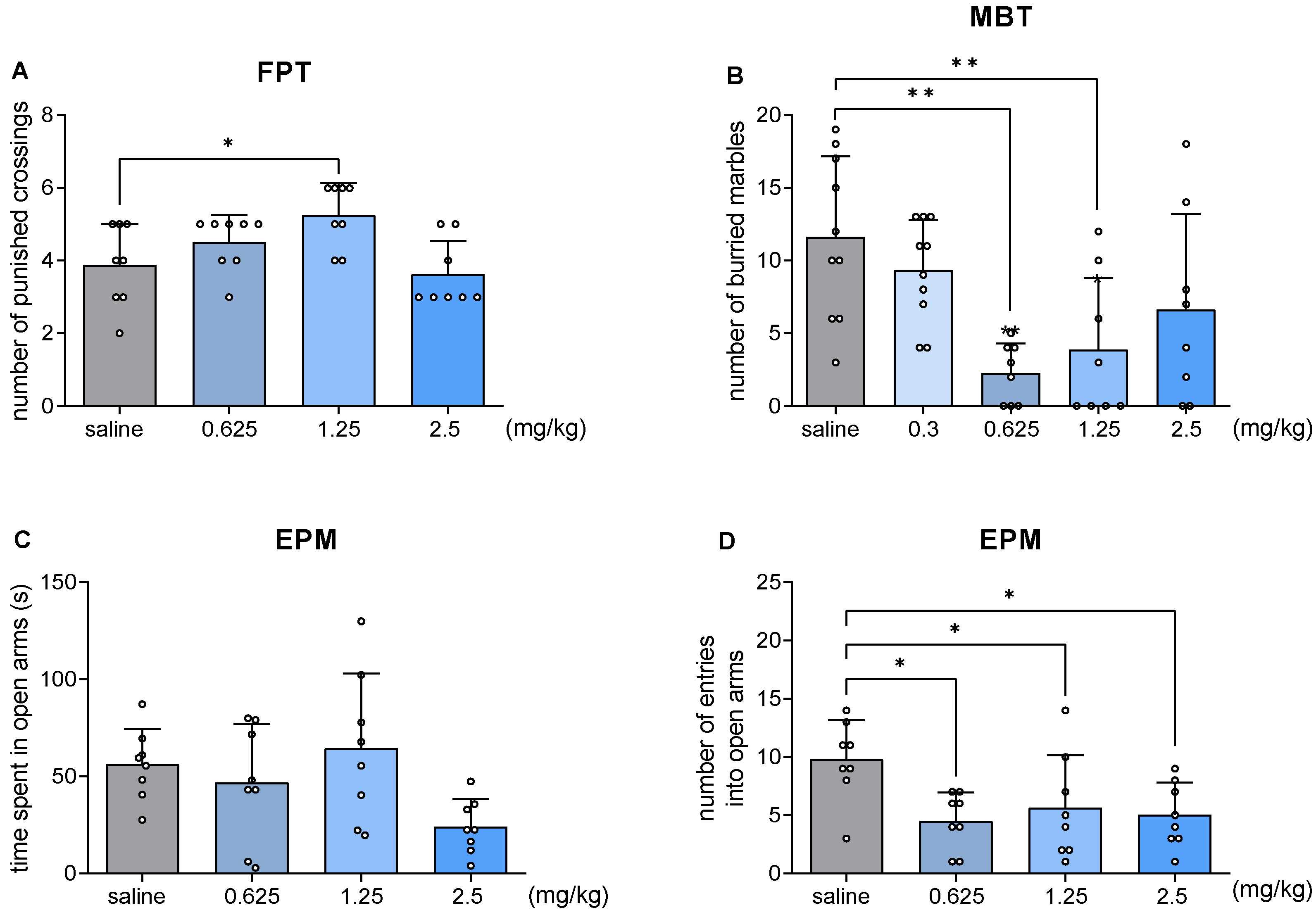
2.4. JJGW07 Increased the Number of Punished Crossings in the Four Plate Test in Mice
2.5. JJGW07 Decreased the Number of Buried Marbles in the Marble-Burying Test in Mice
2.6. JJGW07 Did Not Increase the Time Spent in Open Arms in the Elevated Plus Maze Test in Mice
2.7. JJGW07 Did Not Influence the Locomotor Activity in Mice
2.8. JJGW07 Did Not Influence the Motor Coordination in Mice
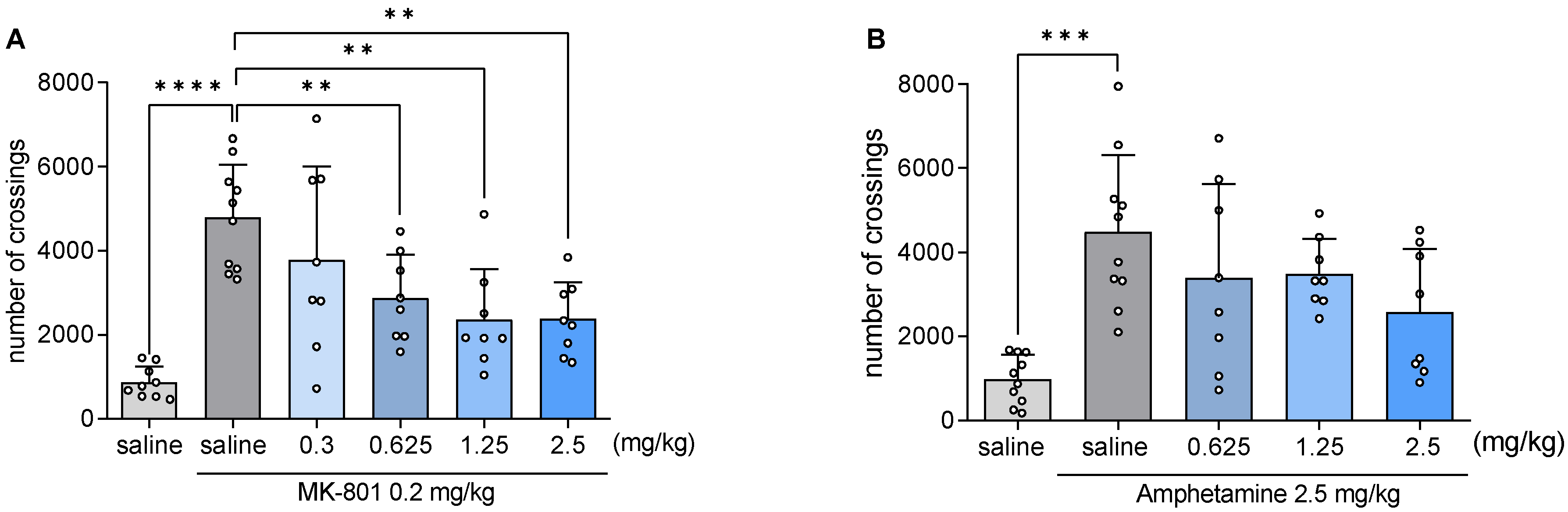
2.9. JJGW07 Reversed the MK-801- and Amphetamine-Induced Hyperlocomotion in Mice
2.10. JJGW07 Did Not Induce Catalepsy in the Bar Test in Mice at Antipsychotic-like Doses
2.11. JJGW07 Did Not Disturb Long-Term Memory in Naïve Mice in the Step-through Passive Avoidance Task
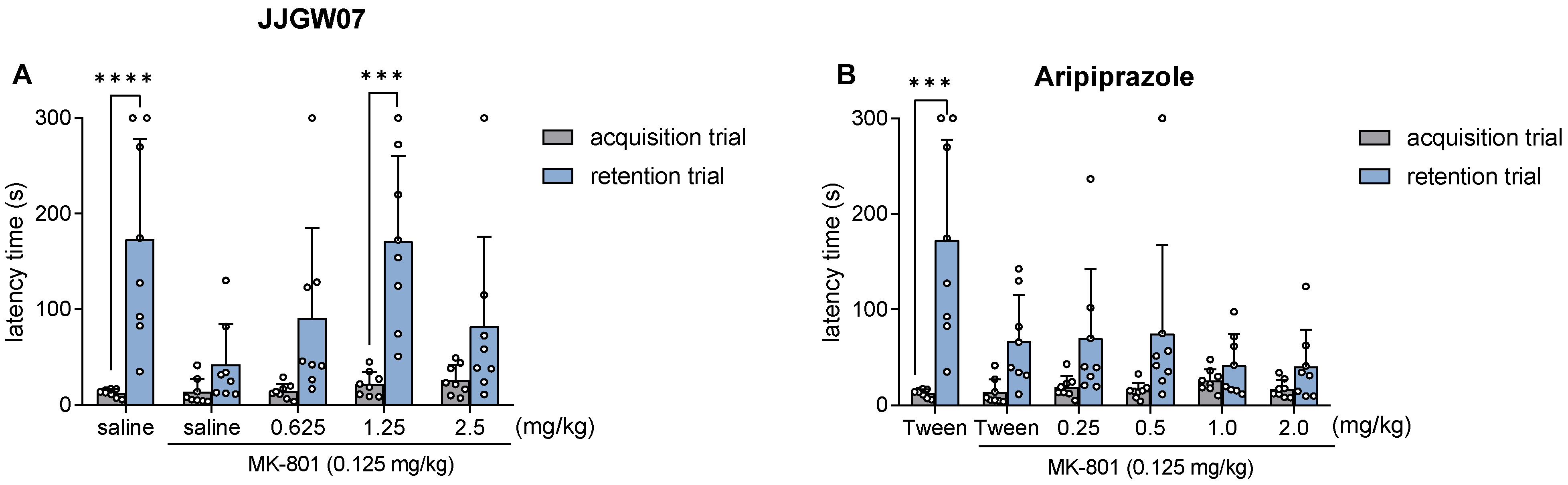
2.12. JJGW07 Reversed Cognitive Disturbances after the MK-801 Administration in Mice in the Step-Through Passive Avoidance Task
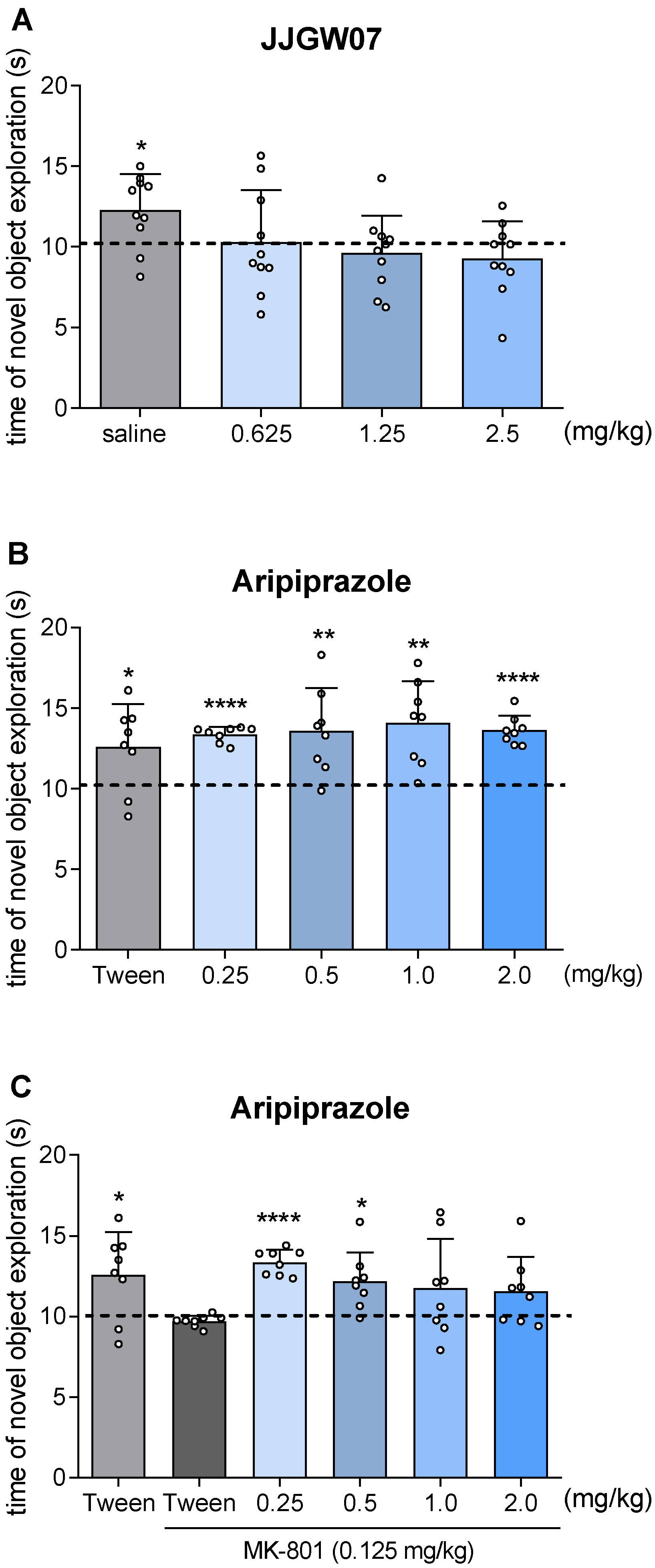
2.13. JJGW07 Did Not Disturb Long-Term Memory in Naïve Mice in the Object Recognition Test
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
4.3. Radioligand Binding Assay
4.4. Functional Assay for 5-HT1A, 5-HT2A, and D2 Receptor
4.5. Functional Assays for 5-HT7 Receptors
4.6. Forced Swim Test
4.7. Tail Suspension Test
4.8. Four-Plate Test
4.9. Marble-Burying Test
4.10. Elevated Plus Maze Test
4.11. Spontaneous Locomotor Activity in Mice
4.12. Rotarod Test
4.13. MK-801- and Amphetamine-Induced Hyperlocomotion Test in Mice
4.14. Catalepsy Bar Test
- -
- Score of 0 points if the animal held the constrained position for <15 s;
- -
- Score of 1 point if the animal stayed on the bar for 15–29.9 s;
- -
- Score of 2 points if the animal stayed on the bar for 30–59.9 s;
- -
- Score of 3 points if the animal stayed on the bar for more than 60 s.
4.15. Step-Through Passive Avoidance Task
4.16. Object Recognition Test
4.17. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, K.; Metcalfe, A.; Bulloch, A.G.M.; Williams, J.V.A.; Patten, S.B. Mental disorders and subsequent suicide events in a representative community population. J. Affect. Disord. 2020, 277, 456–462. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 22 December 2022).
- Gémes, K.; Bergström, J.; Papola, D.; Barbui, C.; Lam, A.I.F.; Hall, B.J.; Seedat, S.; Morina, N.; Quero, S.; Campos, D.; et al. Symptoms of anxiety and depression during the COVID-19 pandemic in six European countries and Australia—Differences by prior mental disorders and migration status. J. Affect. Disord. 2022, 311, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Aljunaidy, M.M.; Adi, M.N. Architecture and Mental Disorders: A Systematic Study of Peer-Reviewed Literature. HERD 2021, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Jaśkowska, J.; Drabczyk, A.K.; Śliwa, P.; Jodłowski, P.; Pindelska, E.; Kułaga, D.; Zaręba, P.; Majka, Z.; Siwek, A.; Wolak, M.; et al. Ultrasound assisted one-pot synthesis and preliminary in vitro studies of salicylamide arylpiperazines as dual 5-HT1A/5-HT7 ligands. J. Mol. Struct. 2022, 1275, 134585. [Google Scholar] [CrossRef]
- Żmudzka, E.; Lustyk, K.; Głuch-Lutwin, M.; Mordyl, B.; Zakrzewska-Sito, A.; Mierzejewski, P.; Jaśkowska, J.; Kołaczkowski, M.; Sapa, J.; Pytka, K. Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents. Int. J. Mol. Sci. 2022, 23, 15929. [Google Scholar] [CrossRef]
- Jamu, I.M.; Okamoto, H. Recent advances in understanding adverse effects associated with drugs targeting the serotonin receptor, 5-HT GPCR. Front. Glob. Womens Health 2022, 3, 1012463. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, Z.; Piao, J.; Cui, R.; Li, B. Dopamine Receptors: Is It Possible to Become a Therapeutic Target for Depression? Front. Pharmacol. 2022, 13, 947785. [Google Scholar] [CrossRef]
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Kurita, J.P.F.; Leão, A.H.F.F.; Bioni, V.S.; Wuo-Silva, R.; Lima, A.C.; Paiva-Santos, M.A.; Marinho, G.F.; Cunha, D.M.G.; Becegato, M.; Lopes-Silva, L.B.; et al. Memory and anxiety-like behavior of rats in the plus-maze discriminative avoidance task: Role of serotonergic transmission in the basolateral amygdala. Behav. Neurosci. 2022. [Google Scholar] [CrossRef]
- Bombardi, C.; Grandis, A.; Pivac, N.; Sagud, M.; Lucas, G.; Chagraoui, A.; Lemaire-Mayo, V.; De Deurwaerdère, P.; Di Giovanni, G. Serotonin modulation of hippocampal functions: From anatomy to neurotherapeutics. Prog. Brain Res. 2021, 261, 83–158. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Feng, J.; Jia, M.; Zhang, G.; Wen, X. Downregulation of 5-hydroxytryptamine7 receptor in the medial prefrontal cortex ameliorates impulsive actions in animal models of schizophrenia. Behav. Brain Res. 2018, 341, 212–223. [Google Scholar] [CrossRef]
- Mallet, J.; Gorwood, P.; Le Strat, Y.; Dubertret, C. Major Depressive Disorder (MDD) and Schizophrenia- Addressing Unmet Needs With Partial Agonists at the D2 Receptor: A Review. Int. J. Neuropsychopharmacol. 2019, 22, 651–664. [Google Scholar] [CrossRef]
- Huang, M.; Kwon, S.; Rajagopal, L.; He, W.; Meltzer, H.Y. 5-HT1A parital agonism and 5-HT7 antagonism restore episodic memory in subchronic phencyclidine-treated mice: Role of brain glutamate, dopamine, acetylcholine and GABA. Psychopharmacology 2018, 235, 2795–2808. [Google Scholar] [CrossRef]
- Lobo, M.C.; Whitehurst, T.S.; Kaar, S.J.; Howes, O.D. New and emerging treatments for schizophrenia: A narrative review of their pharmacology, efficacy and side effect profile relative to established antipsychotics. Neurosci. Biobehav. Rev. 2022, 132, 324–361. [Google Scholar] [CrossRef]
- Spoelstra, S.; Bruggeman, R.; Knegtering, H. An antipsychotic without dopamine receptor blockade? Tijdschr. Psychiatr. 2021, 63, 804–809. [Google Scholar]
- Depoortere, R.Y.; Auclair, A.L.; Newman-Tancredi, A. NLX-101, a cortical 5-HT1A receptor biased agonist, reverses scopolamine-induced deficit in the delayed non-matching to position model of cognition. Brain Res. 2021, 1765, 147493. [Google Scholar] [CrossRef]
- Casey, A.B.; Cui, M.; Booth, R.G.; Canal, C.E. “Selective” serotonin 5-HT2A receptor antagonists. Biochem. Pharmacol. 2022, 200, 115028. [Google Scholar] [CrossRef]
- Okubo, R.; Hasegawa, T.; Fukuyama, K.; Shiroyama, T.; Okada, M. Current Limitations and Candidate Potential of 5-HT7 Receptor Antagonism in Psychiatric Pharmacotherapy. Front. Psychiatry 2021, 12, 623684. [Google Scholar] [CrossRef]
- Solís-Guillén, R.; Leopoldo, M.; Meneses, A.; Centurión, D. Activation of 5-HT1A and 5-HT7 receptors enhanced a positively reinforced long-term memory. Behav. Brain Res. 2021, 397. [Google Scholar] [CrossRef]
- Shahidi, S.; Mahmoodi, M.; Sadeghimehr, N. Involvement of Serotonin 5-HT7 Receptors in Learning and Memory in Mice. Neurophysiol. 2019, 51, 77–82. [Google Scholar] [CrossRef]
- Balcer, O.M.; Seager, M.A.; Gleason, S.D.; Li, X.; Rasmussen, K.; Maxwell, J.K.; Nomikos, G.; Degroot, A.; Witkin, J.M. Evaluation of 5-HT7 receptor antagonism for the treatment of anxiety, depression, and schizophrenia through the use of receptor-deficient mice. Behav. Brain Res. 2019, 360, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin. Schizophr. Relat. Psychoses 2010, 4, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Schizophrenia and dopamine receptors. Eur. Neuropsychopharmacol. 2013, 23, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C. The power issue: Determination of KB or Ki from IC50. A closer look at the Cheng-Prusoff equation, the Schild plot and related power equations. J. Pharmacol. Toxicol. Methods 2001, 46, 61–71. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Ögren, S.O.; Hall, H.; Köhler, C.; Magnusson, O.; Sjöstrand, S.E. The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology 1986, 90, 287–294. [Google Scholar] [CrossRef]
- Vahid-Ansari, F.; Albert, P.R. Rewiring of the Serotonin System in Major Depression. Front. Psychiatry 2021, 12, 802581. [Google Scholar] [CrossRef]
- Geng, F.; Tian, J.; Wu, J.L.; Luo, Y.; Zou, W.J.; Peng, C.; Lu, G.F. Dorsomedial prefrontal cortex 5-HT6 receptors regulate anxiety-like behavior. Cogn. Affect. Behav. Neurosci. 2018, 18, 58–67. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, B.; Chen, C.; Li, C.; Zhang, Y. 5-HT6R null mutatrion induces synaptic and cognitive defects. Aging Cell 2021, 20, e13369. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Okun, I.; Aladinskiy, V.; Ivanenkov, Y.; Koryakova, A.; Karapetyan, R.; Mitkin, O.; Salimov, R.; Ivashchenko, A.; Bezprozvanny, I. AVN-492, A Novel Highly Selective 5-HT6R Antagonist: Preclinical Evaluation. J. Alzheimers Dis. 2017, 58, 1043–1063. [Google Scholar] [CrossRef]
- Andrews, M.; Tousi, B.; Sabbagh, M.N. 5HT6 Antagonists in the Treatment of Alzheimer’s Dementia: Current Progress. Neurol. Ther. 2018, 7, 51. [Google Scholar] [CrossRef]
- Nirogi, R.; Abraham, R.; Benade, V.; Medapati, R.B.; Jayarajan, P.; Bhyrapuneni, G.; Muddana, N.; Mekala, V.R.; Subramanian, R.; Shinde, A.; et al. SUVN-502, a novel, potent, pure, and orally active 5-HT6 receptor antagonist: Pharmacological, behavioral, and neurochemical characterization. Behav. Pharmacol. 2019, 30, 16–35. [Google Scholar] [CrossRef]
- Millan, M.J.; Dekeyne, A.; Gobert, A.; Brocco, M.; Mannoury la Cour, C.; Ortuno, J.C.; Watson, D.; Fone, K.C.F. Dual-acting agents for improving cognition and real-world function in Alzheimer’s disease: Focus on 5-HT6 and D3 receptors as hubs. Neuropharmacology 2020, 177. [Google Scholar] [CrossRef]
- Sudoł, S.; Cios, A.; Jastrzębska-więsek, M.; Honkisz-orzechowska, E.; Mordyl, B.; Wilczyńska-zawal, N.; Satała, G.; Kucwaj-brysz, K.; Partyka, A.; Latacz, G.; et al. The phenoxyalkyltriazine antagonists for 5-ht6 receptor with promising procognitive and pharmacokinetic properties in vivo in search for a novel therapeutic approach to dementia diseases. Int. J. Mol. Sci. 2021, 22, 10773. [Google Scholar] [CrossRef]
- Hirano, K.; Searle, K.L.; Nasir, S.; Aw, C.C.; Browne, E.R.; Rutter, A.R. In vivo 5-HT(6) receptor occupancy by antipsychotic drugs in the rat brain. Neurosci. Lett. 2011, 503, 240–243. [Google Scholar] [CrossRef]
- Zlatanova, H.I.; Georgieva-Kotetarova, M.T.; Vilmosh, N.B.; Kandilarov, I.K. Evaluation of the Effect of Cariprazine on Memory and Cognition in Experimental Rodent Models. Int. J. Environ. Res. Public Health 2022, 19, 14748. [Google Scholar] [CrossRef]
- Riedel, M.; Spellmann, I.; Schennach-Wolff, R.; Musil, R.; Dehning, S.; Cerovecki, A.; Opgen-Rhein, M.; Matz, J.; Seemüller, F.; Obermeier, M.; et al. Effect of aripiprazole on cognition in the treatment of patients with schizophrenia. Pharmacopsychiatry 2010, 43, 50–57. [Google Scholar] [CrossRef]
- Orzelska-Górka, J.; Mikulska, J.; Wiszniewska, A.; Biała, G. New Atypical Antipsychotics in the Treatment of Schizophrenia and Depression. Int. J. Mol. Sci. 2022, 23, 10624. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Pytka, K.; Zmudzka, E.; Lustyk, K.; Rapacz, A.; Olczyk, A.; Galuszka, A.; Waszkielewicz, A.; Marona, H.; Sapa, J.; Barbara, F. The antidepressant- and anxiolytic-like activities of new xanthone derivative with piperazine moiety in behavioral tests in mice. Indian J. Pharmacol. 2016, 48, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.M.; Sanz, G.; Vaz, B.G.; de Carvalho, F.S.; Lião, L.M.; de Oliveira, D.R.; da Silva Moreira, L.K.; Cardoso, C.S.; de Brito, A.F.; da Silva, D.P.B.; et al. Tert-butyl 4-((1-phenyl-1H-pyrazol-4-yl) methyl) piperazine-1-carboxylate (LQFM104)- New piperazine derivative with antianxiety and antidepressant-like effects: Putative role of serotonergic system. Biomed. Pharmacother. 2018, 103, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Partyka, A.; Jastrzębska-Więsek, M.; Siwek, A.; Głuch-Lutwin, M.; Mordyl, B.; Kazek, G.; Rapacz, A.; Olczyk, A.; Gałuszka, A.; et al. Antidepressant- and Anxiolytic-Like Effects of New Dual 5-HT1A and 5-HT7 Antagonists in Animal Models. PLoS ONE 2015, 10, e0142499. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Kazek, G.; Siwek, A.; Mordyl, B.; Głuch-Lutwin, M.; Rapacz, A.; Olczyk, A.; Gałuszka, A.; Waszkielewicz, A.; Marona, H.; et al. HBK-7—A new xanthone derivative and a 5-HT1A receptor antagonist with antidepressant-like properties. Pharmacol. Biochem. Behav. 2016, 146–147, 35–43. [Google Scholar] [CrossRef]
- Partyka, A.; Chłoń-Rzepa, G.; Wasik, A.; Jastrzębska-Więsek, M.; Bucki, A.; Kołaczkowski, M.; Satała, G.; Bojarski, A.J.; Wesołowska, A. Antidepressant- and anxiolytic-like activity of 7-phenylpiperazinylalkyl-1,3-dimethyl-purine-2,6-dione derivatives with diversified 5-HT1A receptor functional profile. Bioorg. Med. Chem. 2015, 23, 212–221. [Google Scholar] [CrossRef]
- Wesołowska, A.; Paluchowska, M.H.; Gołembiowska, K.; Chojnacka-Wójcik, E. Pharmacological characterization of MP349, a novel 5-HT1A-receptor antagonist with anxiolytic-like activity, in mice and rats. J. Pharm. Pharmacol. 2003, 55, 533–543. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Stachowicz, K.; Tatarczyńska, E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 2006, 51, 578–586. [Google Scholar] [CrossRef]
- Maxwell, J.; Gleason, S.D.; Falcone, J.; Svensson, K.; Balcer, O.M.; Li, X.; Witkin, J.M. Effects of 5-HT7 receptor antagonists on behaviors of mice that detect drugs used in the treatment of anxiety, depression, or schizophrenia. Behav. Brain Res. 2019, 359, 467–473. [Google Scholar] [CrossRef]
- Lustyk, K.; Sałaciak, K.; Jakubczyk, M.; Jastrzębska-Więsek, M.; Partyka, A.; Wesołowska, A.; Marona, H.; Pytka, K. HBK-15, a Multimodal Compound, Showed an Anxiolytic-Like Effect in Rats. Neurochem. Res. 2022. [Google Scholar] [CrossRef]
- Pytka, K.; Głuch-Lutwin, M.; Zmudzka, E.Z.; Sałaciak, K.; Siwek, A.; Niemczyk, K.; Walczak, M.; Smolik, M.; Olczyk, A.; Gałuszka, A.; et al. HBK-17, a 5-HT1A Receptor Ligand With Anxiolytic-Like Activity, Preferentially Activates ß-Arrestin Signaling. Front. Pharmacol. 2018, 9, 1146. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef]
- Deacon, R.M.J.; Rawlins, J.N.P. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav. Brain Res. 2005, 156, 241–249. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef]
- Watzman, N.; Barry, H. Drug effects on motor coordination. Psychopharmacologia 1968, 12, 414–423. [Google Scholar] [CrossRef]
- Strömberg, C. Interactions of antidepressants and ethanol on spontaneous locomotor activity and rotarod performance in NMRI and C57BL/6 mice. J. Psychopharmacol. 1988, 2, 61–66. [Google Scholar] [CrossRef]
- Holthoewer, D.; Kirschbaum, K.M.; Frisch, J.; Hiemke, C.; Schmitt, U. Pharmacodynamic effects of aripiprazole and ziprasidone with respect to p-glycoprotein substrate properties. Pharmacopsychiatry 2013, 46, 175–180. [Google Scholar] [CrossRef]
- Refsgaard, L.K.; Pickering, D.S.; Andreasen, J.T. Investigation of antidepressant-like and anxiolytic-like actions and cognitive and motor side effects of four N-methyl-D-aspartate receptor antagonists in mice. Behav. Pharmacol. 2017, 28, 37–47. [Google Scholar] [CrossRef]
- Chokhawala, K.; Stevens, L. Antipsychotic Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Preda, A.; Shapiro, B.B. A safety evaluation of aripiprazole in the treatment of schizophrenia. Expert Opin. Drug Saf. 2020, 19, 1529–1538. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A.; Patel, D.; Patel, K.; Bambharoliya, T. Mini Review on Cariprazine: A Promising Antipsychotic Agent. CNS Neurol. Disord. Drug Targets 2023, 22, 226–236. [Google Scholar] [CrossRef]
- Correll, C.U. Current Treatment Options and Emerging Agents for Schizophrenia. J. Clin. Psychiatry 2020, 81. [Google Scholar] [CrossRef]
- Krogmann, A.; Peters, L.; Von Hardenberg, L.; Bödeker, K.; Nöhles, V.B.; Correll, C.U. Keeping up with the therapeutic advances in schizophrenia: A review of novel and emerging pharmacological entities. CNS Spectr. 2019, 24, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.J.; Fadayel, G.M. Regional effects of MK-801 on dopamine release: Effects of competitive NMDA or 5-HT2A receptor blockade. J. Pharmacol. Exp. Ther. 1996, 277. [Google Scholar]
- Ferrucci, M.; Limanaqi, F.; Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Fornai, F. The effects of amphetamine and methamphetamine on the release of norepinephrine, dopamine and acetylcholine from the brainstem reticular formation. Front. Neuroanat. 2019, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.E. Neuroleptic-Induced Acute Extrapyramidal Syndromes and Tardive Dyskinesia. Psychiatr. Clin. N. Am. 1993, 16, 589–610. [Google Scholar] [CrossRef]
- Sykes, D.A.; Moore, H.; Stott, L.; Holliday, N.; Javitch, J.A.; Robert Lane, J.; Charlton, S.J. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat. Commun. 2017, 8, 763. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Hooten, W.M. Extrapyramidal Symptoms. Clin. Nurse Spec. 2022, 7, 224. [Google Scholar] [CrossRef]
- Blair, D.T.; Dauner, A. Extrapyramidal symptoms are serious side-effects of antipsychotic and other drugs. Nurse Pract. 1992, 17, 56–67. [Google Scholar] [CrossRef]
- Aleman, A.; Hijman, R.; De Haan, E.H.F.; Kahn, R.S. Memory impairment in schizophrenia: A meta-analysis. Am. J. Psychiatry 1999, 156, 1358–1366. [Google Scholar] [CrossRef]
- Guo, J.Y.; Ragland, J.D.; Carter, C.S. Memory and Cognition in Schizophrenia. Mol. Psychiatry 2019, 24, 633. [Google Scholar] [CrossRef]
- Forbes, N.F.; Carrick, L.A.; McIntosh, A.M.; Lawrie, S.M. Working memory in schizophrenia: A meta-analysis. Psychol. Med. 2009, 39, 889–905. [Google Scholar] [CrossRef]
- Burriss, L.; Ayers, E.; Ginsberg, J.; Powell, D.A. Learning and memory impairment in PTSD: Relationship to depression. Depress. Anxiety 2008, 25, 149–157. [Google Scholar] [CrossRef]
- Cordani, C.; Young, V.M.; Arienti, C.; Lazzarini, S.G.; Del Furia, M.J.; Negrini, S.; Kiekens, C. Cognitive impairment, anxiety and depression: A map of Cochrane evidence relevant to rehabilitation for people with post COVID-19 condition. Eur. J. Phys. Rehabil. Med. 2022. [Google Scholar] [CrossRef]
- Schweizer, S.; Kievit, R.A.; Emery, T.; Henson, R.N. Symptoms of depression in a large healthy population cohort are related tosubjective memory complaints and memory performance in negative contexts. Psychol. Med. 2018, 48, 104. [Google Scholar] [CrossRef]
- Botto, R.; Callai, N.; Cermelli, A.; Causarano, L.; Rainero, I. Anxiety and depression in Alzheimer’s disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol. Sci. 2022, 43, 4107–4124. [Google Scholar] [CrossRef]
- Kizilbash, A.H.; Vanderploeg, R.D.; Curtiss, G. The effects of depression and anxiety on memory performance. Arch. Clin. Neuropsychol. 2002, 17, 57–67. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Sałaciak, K.; Malikowska-Racia, N.; Lustyk, K.; Siwek, A.; Głuch-Lutwin, M.; Kazek, G.; Popiół, J.; Sapa, J.; Marona, H.; Żelaszczyk, D.; et al. Synthesis and Evaluation of the Antidepressant-like Properties of HBK-10, a Novel 2-Methoxyphenylpiperazine Derivative Targeting the 5-HT1A and D2 Receptors. Pharmaceuticals 2021, 14, 744. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Kołaczkowski, M.; Marcinkowska, M.; Bucki, A.; Pawłowski, M.; Mitka, K.; Jaśkowska, J.; Kowalski, P.; Kazek, G.; Siwek, A.; Wasik, A.; et al. Novel arylsulfonamide derivatives with 5-HT6/5-HT7 receptor antagonism targeting behavioral and psychological symptoms of dementia. J. Med. Chem. 2014, 57, 4543–4557. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Pytka, K.; Socała, K.; Rapacz, A.; Nieoczym, D.; Pieróg, M.; Gryboś, A.; Siwek, A.; Waszkielwicz, A.; Wlaź, P. HBK-14 and HBK-15, triple 5-HT1A, 5-HT7 and 5-HT3 antagonists with potent antidepressant- and anxiolytic-like properties, increase seizure threshold in various seizure tests in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Aron, C.; Simon, P.; Larousse, C.; Boissier, J.R. Evaluation of a rapid technique for detecting minor tranquilizers. Neuropharmacology 1971, 10, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Masse, F.; Dailly, E.; Hascoët, M. Anxiolytic-like effect of milnacipran in the four-plate test in mice: Mechanism of action. Pharmacol. Biochem. Behav. 2005, 81, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Broekkamp, C.L.; Rijk, H.W.; Joly-Gelouin, D.; Lloyd, K.L. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur. J. Pharmacol. 1986, 126, 223–229. [Google Scholar] [CrossRef]
- Pytka, K.; Rapacz, A.; Zygmunt, M.; Olczyk, A.; Waszkielewicz, A.; Sapa, J.; Filipek, B. Antidepressant-like activity of a new piperazine derivative of xanthone in the forced swim test in mice: The involvement of serotonergic system. Pharmacol. Rep. 2015, 67, 160–165. [Google Scholar] [CrossRef]
- Carlsson, M.L.; Martin, P.; Nilsson, M.; Sorensen, S.M.; Carlsson, A.; Waters, S.; Waters, N. The 5-HT2A receptor antagonist M100907 is more effective in counteracting NMDA antagonist- than dopamine agonist-induced hyperactivity in mice. J. Neural Transm. 1999, 106, 123–129. [Google Scholar] [CrossRef]
- Nilsson, M.; Carlsson, A.; Carlsson, M.L. Glycine and D-serine decrease MK-801-induced hyperactivity in mice. J. Neural Transm. 1997, 104, 1195–1205. [Google Scholar] [CrossRef]
- Ueki, S.; Fujiwara, M.; Inoue, K.; Kataoka, Y.; Ibii, N.; Wada, Y. Behavior pharmacology of maprotiline, a new antidepressant. Nihon Yakurigaku Zasshi 1975, 71, 789–815. [Google Scholar] [CrossRef]
- Czopek, A.; Kołaczkowski, M.; Bucki, A.; Byrtus, H.; Pawłowski, M.; Kazek, G.; Bojarski, A.J.; Piaskowska, A.; Kalinowska-Tłücik, J.; Partyka, A.; et al. Novel spirohydantoin derivative as a potent multireceptor-active antipsychotic and antidepressant agent. Bioorg. Med. Chem. 2015, 23, 3436–3447. [Google Scholar] [CrossRef]
- Lee, H.E.; Jeon, S.J.; Ryu, B.; Park, S.J.; Ko, S.Y.; Lee, Y.; Kim, E.; Lee, S.; Kim, H.; Jang, D.S.; et al. Swertisin, a C-glucosylflavone, ameliorates scopolamine-induced memory impairment in mice with its adenosine A1 receptor antagonistic property. Behav. Brain Res. 2016, 306, 137–145. [Google Scholar] [CrossRef]
- Bevins, R.A.; Besheer, J. Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study “recognition memory”. Nat. Protoc. 2006, 1, 1306–1311. [Google Scholar] [CrossRef]

| Receptor | Treatment | Agonist Mode | Antagonist Mode | ||||
|---|---|---|---|---|---|---|---|
| Emax% | pEC50 ± Range | Emax% | pIC50 ± Range | Kb (nM) | R2Kb | ||
| Quiniprol | 100 | 8.70 ± 0.12 | 0 | n.c. | n.c. | n.c. | |
| D2 | Apomorphine | 100 | 7.50 ± 0.08 | 0 | n.c. | n.c. | n.c. |
| Chlorpromazine | 2 | n.c. | 0 | 9.78 ± 0.42 | 0.03 | 0.94 | |
| JJGW07 | 20 | n.c. | 3 | 7.03 ± 0.01 | 18 | 0.86 | |
| Serotonin | 100 | 7.63 ± 1.05 | 0 | n.c. | n.c | n.c. | |
| 5-HT1A | NAN-190 | 6 | n.c. | 0 | 8.98 ± 0.05 | 0.07 | 0.99 |
| JJGW07 | 1 | n.c | 0 | 7.38 ± 0.52 | 3 | 0.85 | |
| α-methyloserotonin | 100 | 8.50 ± 0.31 | 2 | n.c. | n.c. | n.c. | |
| 5-HT2A | Serotonin | 112 | 8.36 ± 0.04 | 1 | n.c. | n.c. | n.c. |
| Mianserin | 3 | n.c. | 3 | 8.07 ± 0.08 | 2.3 | 0.91 | |
| JJGW07 | 28 | 5.81 ± 0.29 | 17 | 5.56 ± 0.87 | 550 | 0.92 | |
| Serotonin | 100 | 8.06 ± 0.14 | 0 | n.c. | n.c. | n.c. | |
| 5-HT7 | SB-269970 | 0 | n.c. | 9.0 | 9.29 ± 0.21 | 0.2 | 0.94 |
| JJGW07 | 1 | n.c. | 6.0 | 5.46 ± 0.34 | 1600 | 0.99 | |
| Treatment | Dose (mg/kg) | Number of Crossings ± SD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 min | 30 min | 6 min | 5 min | 4 min | 1 min | ||||||||||||||
| Saline | - | 2112 | ± | 1156 | 773 | ± | 578 | 274 | ± | 106 | 198 | ± | 102 | 184 | ± | 81 | 38 | ± | 24 |
| 0.08 | - | - | 226 | 37 | - | - | - | ||||||||||||
| 0.15 | - | - | 325 | ± | 84 | - | - | - | |||||||||||
| JJGW07 | 0.3 | - | 1133 | ± | 224 | 305 | ± | 67 | - | - | - | ||||||||
| 0.625 | 2247 | ± | 362 | 806 | ± | 289 | 237 | ± | 49 | 193 | ± | 35 | 161 | ± | 51 | 32 | ± | 11 | |
| 1.25 | 2029 | ± | 781 | 1248 | ± | 441 | 232 | ± | 75 | 203 | ± | 73 | 141 | ± | 78 | 35 | ± | 20 | |
| 2.5 | 3035 | ± | 962 | 884 | ± | 262 | 270 | ± | 50 | 214 | ± | 62 | 179 | ± | 72 | 42 | ± | 20 | |
| Treatment | Dose (mg/kg) | Animals That Fell from the Rotating Rod | Time before Animals Fell (s) | TD50 (mg/kg) |
|---|---|---|---|---|
| JJGW07 | 20 | 2/8 | 58 ± 3 | 28.3 (22.6–35.4) |
| 30 | 3/8 | 50 ± 17 | ||
| 40 | 7/8 | 20 ± 24 |
| Treatment | Dose (mg/kg) | Mean Score | ||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | ||
| 5 | 0.0 | 0.0 | 0.0 | |
| JJGW07 | 10 | 1.0 | 1.0 | 1.0 |
| 20 | 1.3 | 0.4 | 0.1 |
| Receptor | Radioligand/Final Concentration | Blank (Non-Specific) | Buffer | Incubation Conditions |
|---|---|---|---|---|
| 5-HT6 | [3H]-LSD 2 nM | 10 µM methiothepine | 50 mM Tris–HCl pH 7.4 0.5 mM EDTA, 4 mM MgCl2 | 60 min, 37 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmudzka, E.; Lustyk, K.; Głuch-Lutwin, M.; Wolak, M.; Jaśkowska, J.; Kołaczkowski, M.; Sapa, J.; Pytka, K. Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice. Pharmaceuticals 2023, 16, 175. https://doi.org/10.3390/ph16020175
Żmudzka E, Lustyk K, Głuch-Lutwin M, Wolak M, Jaśkowska J, Kołaczkowski M, Sapa J, Pytka K. Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice. Pharmaceuticals. 2023; 16(2):175. https://doi.org/10.3390/ph16020175
Chicago/Turabian StyleŻmudzka, Elżbieta, Klaudia Lustyk, Monika Głuch-Lutwin, Małgorzata Wolak, Jolanta Jaśkowska, Marcin Kołaczkowski, Jacek Sapa, and Karolina Pytka. 2023. "Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice" Pharmaceuticals 16, no. 2: 175. https://doi.org/10.3390/ph16020175
APA StyleŻmudzka, E., Lustyk, K., Głuch-Lutwin, M., Wolak, M., Jaśkowska, J., Kołaczkowski, M., Sapa, J., & Pytka, K. (2023). Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice. Pharmaceuticals, 16(2), 175. https://doi.org/10.3390/ph16020175





