Development of a Three-Dimensional Nanostructure SnO2-Based Gas Sensor for Room-Temperature Hydrogen Detection
Abstract
1. Introduction
2. Materials and Methods
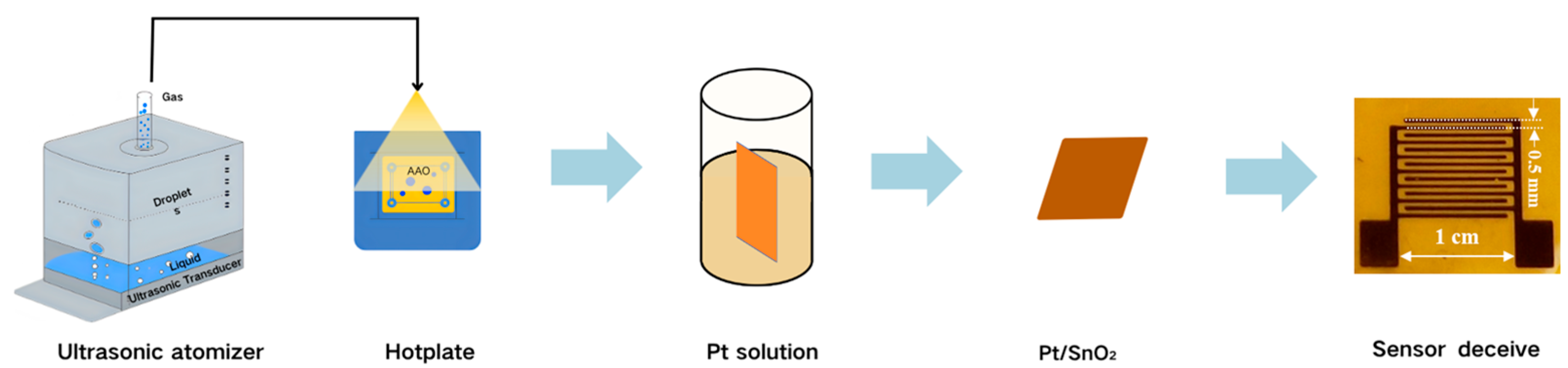
2.1. Fabrication of Pt/SnO2 Sensor
2.2. Characterization
2.3. Gas Sensing Measurements
3. Results and Discussion
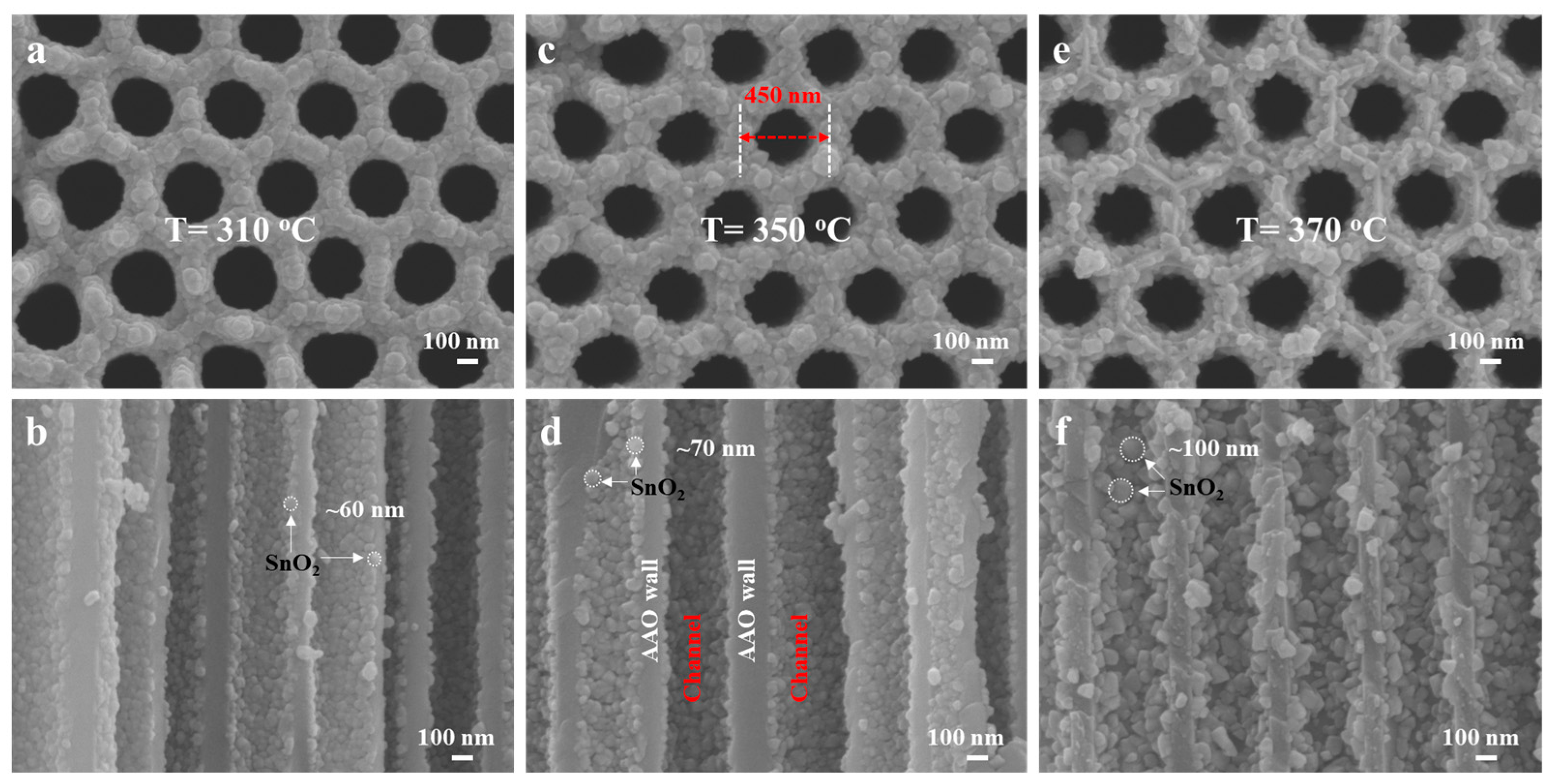
3.1. Fabrication and Characterization of the Sensors
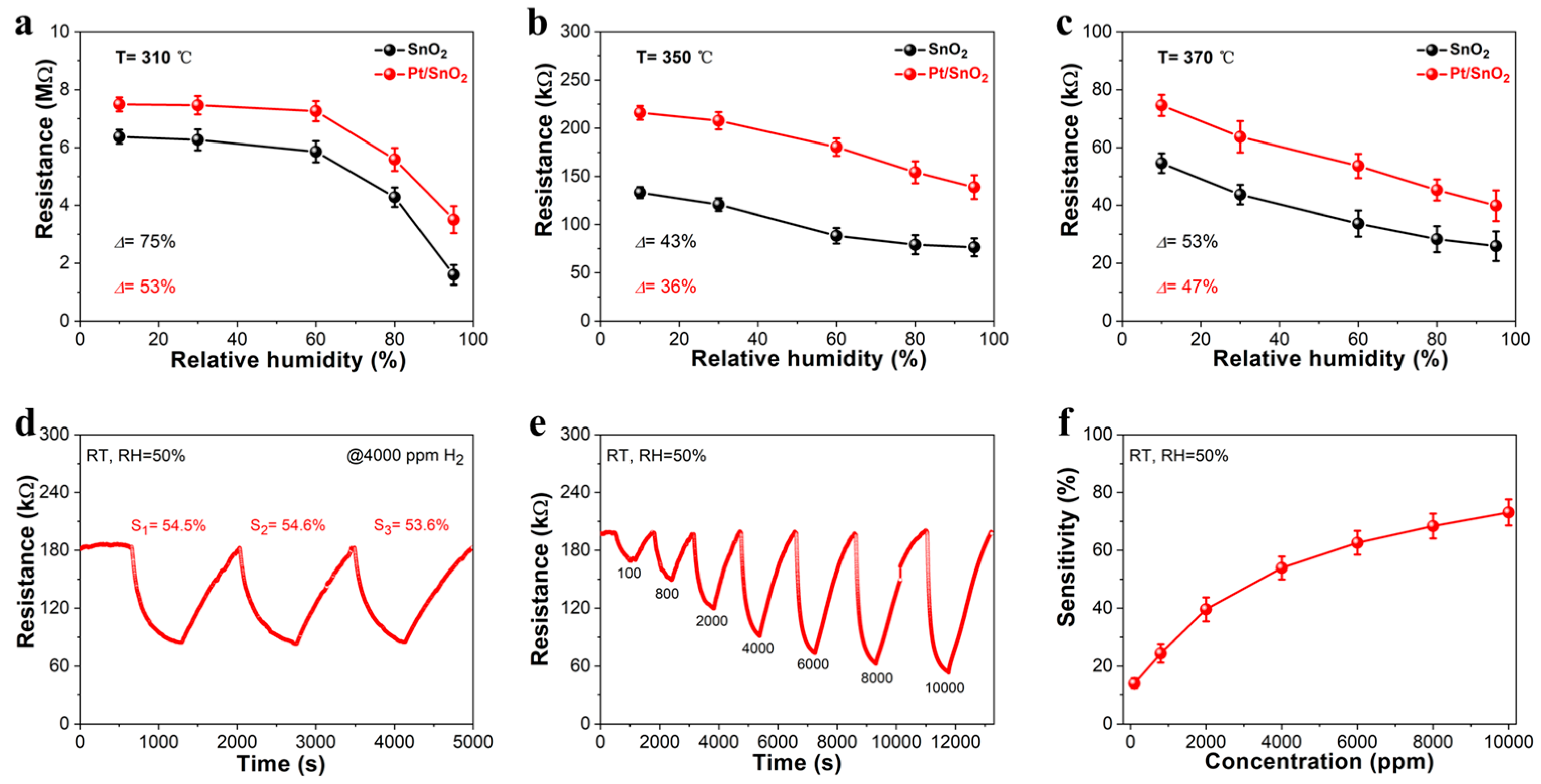
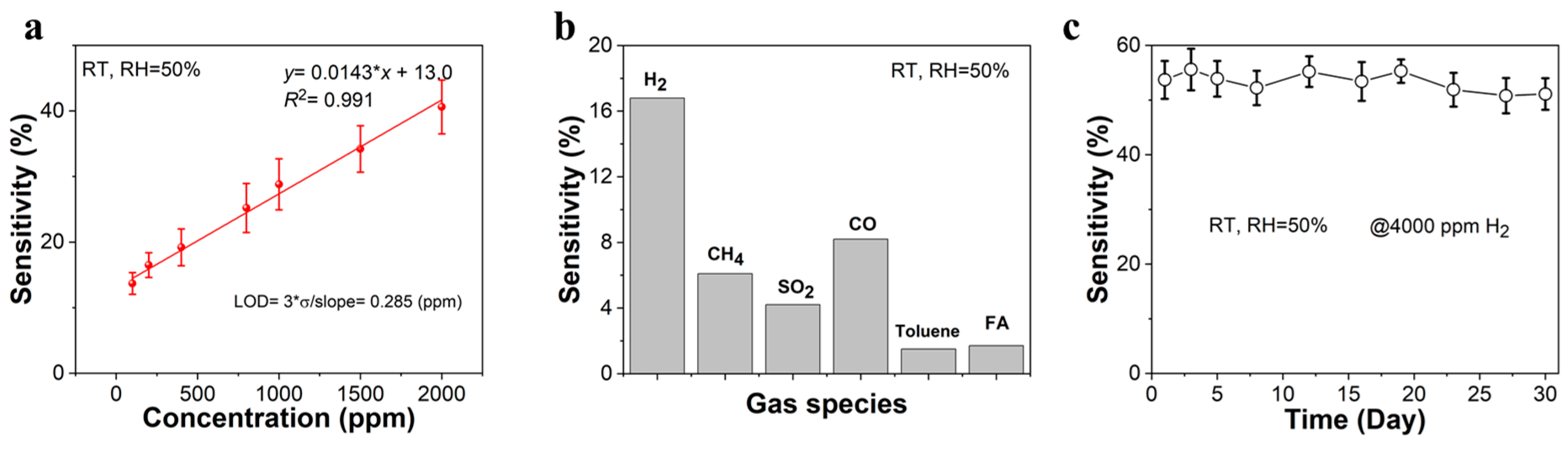
3.2. Gas-Sensing Properties
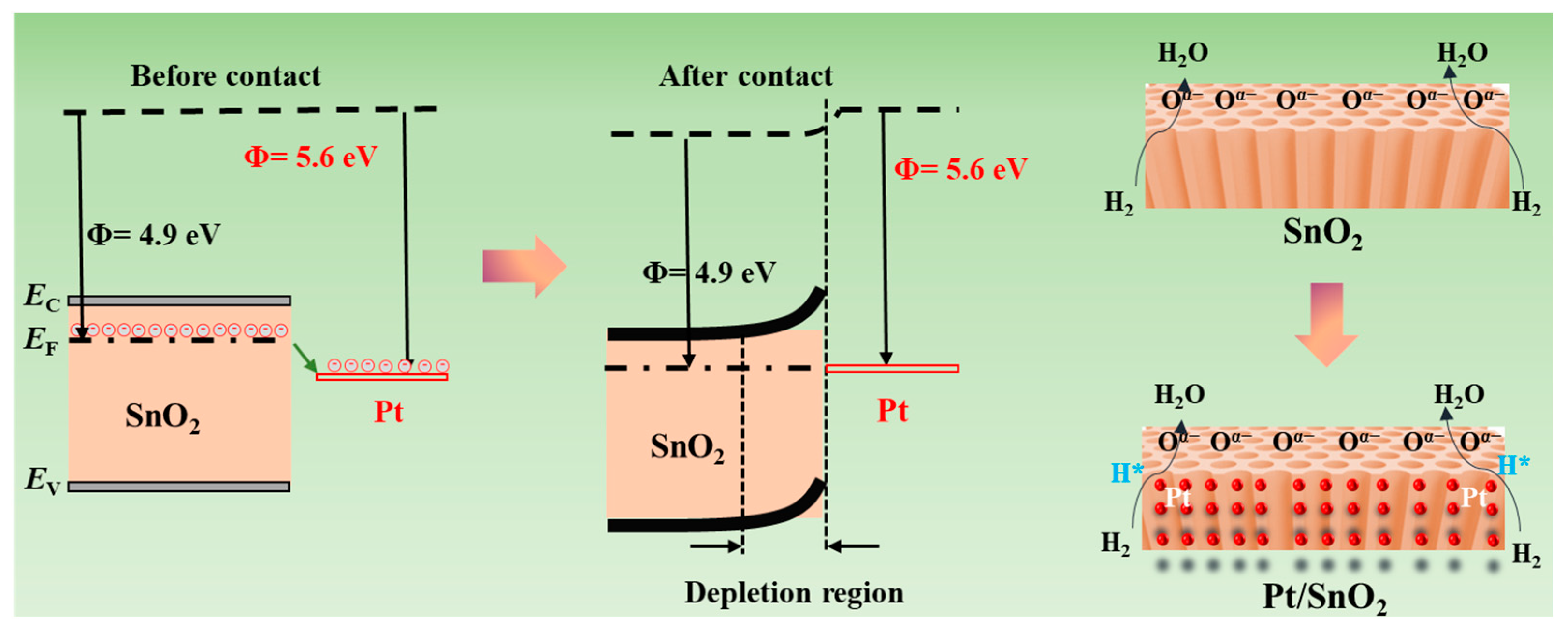
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, Q.R.; Zhang, T.; Zhu, Z.H.; Cai, R.; Song, K.; Yan, F.; Qayyum, A. Harnessing hydrogen energy storage for renewable energy stability in China: A path to carbon neutrality. Int. J. Hydrogen Energy 2025, 118, 93–101. [Google Scholar] [CrossRef]
- Hui, Y.Z.; Wang, M.T.; Guo, S.R.; Akhtar, S.; Bhattacharya, S.; Dai, B.; Yu, J. Comprehensive review of development and applications of hydrogen energy technologies in China for carbon neutrality: Technology advances and challenges. Energy Convers. Manag. 2024, 315, 118776. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Barhoumi, E.M.; Belgacem, I.B.; Mansir, I.B.; Aliyu, M.; Emori, W.; Uzoma, P.C.; Beitelmal, W.H.; Akyüz, E.; Radwan, A.B.; et al. A focused review of the hydrogen storage tank embrittlement mechanism process. Int. J. Hydrogen Energy 2023, 48, 12935–12948. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, Y.X.; Shi, J.Y.; Li, Z.H.; Wang, X.; Hu, X.T.; Gong, Y.Y.; Zou, X.B. A Novel Gas Sensor for Detecting Pork Freshness Based on PANI/AgNWs/Silk. Foods 2022, 11, 2372. [Google Scholar] [CrossRef]
- Yin, L.M.; Jayan, H.; Cai, J.R.; El-Seedi, H.R.R.; Guo, Z.M.; Zou, X.B. Spoilage Monitoring and Early Warning for Apples in Storage Using Gas Sensors and Chemometrics. Foods 2023, 12, 2968. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ahmad, W.; Rong, Y.N.; Chen, X.Y.; Zhao, S.G.; Yu, J.H.; Zheng, P.F.; Huang, C.C.; Li, H.H. A Gas Sensors Detection System for Real-Time Monitoring of Changes in Volatile Organic Compounds during Oolong Tea Processing. Foods 2024, 13, 1721. [Google Scholar] [CrossRef]
- Mao, H.P.; Du, X.X.; Yan, Y.T.; Zhang, X.D.; Ma, G.X.; Wang, Y.F.; Liu, Y.; Wang, B.; Yang, X.Y.; Shi, Q. Highly sensitive detection of daminozide using terahertz metamaterial sensors. Int. J. Agric. Biol. Eng. 2022, 15, 180–188. [Google Scholar] [CrossRef]
- Huang, X.W.; Sun, W.; Li, Z.H.; Shi, J.Y.; Zhang, N.; Zhang, Y.; Zhai, X.D.; Hu, X.T.; Zou, X.B. Hydrogen sulfide gas sensing toward on-site monitoring of chilled meat spoilage based on ratio-type fluorescent probe. Food Chem. 2022, 396, 133654. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhou, X.C.; Shi, J.Y.; Zou, X.B.; Huang, X.W.; Tahir, H.E. Preparation of conducting polyaniline/protoporphyrin composites and their application for sensing VOCs. Food Chem. 2019, 276, 291–297. [Google Scholar] [CrossRef]
- Alaghmandfard, A.H.; Fardindoost, S.; Frencken, A.L.; Hoorfar, M. The next generation of hydrogen gas sensors based on transition metal dichalcogenide-metal oxide semiconductor hybrid structures. Ceram. Int. 2024, 50, 29026–29043. [Google Scholar] [CrossRef]
- Kafil, V.; Sreenan, B.; Hadj-Nacer, M.; Wang, Y.; Yoon, J.; Greiner, M.; Chu, P.; Wang, X.; Fadali, M.S.; Zhu, X. Review of noble metal and metal-oxide-semiconductor based chemiresistive hydrogen sensors. Sens. Actuators A Phys. 2024, 373, 115440. [Google Scholar] [CrossRef]
- Song, Z.; Yan, J. Unveiling the Doping Effect of Sub-4 nm Ultrathin SnO2 Quantum Wires on Gas Sensors. Chem. Mater. 2023, 35, 7750–7760. [Google Scholar] [CrossRef]
- Song, Z.; Fang, W.; Zhu, B.; Yan, J. Nano-Schottky-junction-engineered Pd/SnO2 Nanotube Array for Ultrasensitive Hydrogen Sensing at Room Temperature. Anal. Methods 2024, 16, 5954–5958. [Google Scholar] [CrossRef]
- Yan, J.; Song, Z. Metal-decorated 3D Tin Oxide Nanotubes in a Monolithic Sensor Array Chip for Room-Temperature Gas Identification. J. Alloys Compd. 2024, 976, 173075. [Google Scholar] [CrossRef]
- Lv, J.X.; Zhang, C.N.; Qu, G.F.; Pan, K.H.; Qin, J.; Wei, K.L.; Liang, Y.Q. Modification strategies for semiconductor metal oxide nanomaterials applied to chemiresistive NOX gas sensors: A review. Talanta 2024, 273, 125853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Yuan, Z.Y.; Zhu, H.M.; Wang, B.; Wang, H.; Meng, F. Study on the impact of minor ambient temperature variations on the gas sensing performance of zinc cobaltate semiconductor metal oxide sensor for toluene detection. Sens. Actuators B Chem. 2025, 433, 137570. [Google Scholar] [CrossRef]
- Prasertying, P.; Nacapricha, D.; Silvester, D.S. 3D nanoporous-structured Gold/Copper on microelectrode arrays for enhanced gas sensing in room temperature ionic liquids. Electrochim. Acta 2025, 530, 146392. [Google Scholar] [CrossRef]
- Lv, L.; Wang, Y.; Cheng, P.; Zhang, B.; Dang, F.; Xu, L. Ultrasonic spray pyrolysis synthesis of three-dimensional ZnFe2O4-based macroporous spheres for excellent sensitive acetone gas sensor. Sens. Actuators B Chem. 2019, 297, 126755. [Google Scholar] [CrossRef]
- Sriram, S.R.; Parne, S.R.; Pothukanuri, N.; Edla, D.R. Prospects of spray pyrolysis technique for gas sensor applications—A comprehensive review. J. Anal. Appl. Pyrolysis 2022, 164, 105527. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y. Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors. Materials 2022, 15, 8728. [Google Scholar] [CrossRef]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Mou, H.R.; Sun, Y.; Zeng, Z.G.; Zhao, H.B. Low-temperature hydrogen detection sensor based on CeO2-doped SnO2. J. Mater. Sci. Mater. Electron. 2020, 31, 15785–15793. [Google Scholar] [CrossRef]
- Zhang, X.X.; Sun, J.H.; Tang, K.S.; Wang, H.; Chen, T.; Jiang, K.; Zhou, T.; Quan, H.; Guo, R. Ultralow detection limit and ultrafast response/recovery of the H2 gas sensor based on Pd-doped rGO/ZnO-SnO2 from hydrothermal synthesis. Microsyst. Nanoeng. 2022, 8, 67. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, L.; Xu, X.; Xiang, J.; Huang, S.; Lu, T.; Hou, X. Ce Doping Effects on the Hydrogen Sensing Properties of Graphene/SnO2-Based Sensors. Materials 2024, 17, 4382. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Z.; Zhao, L.; Sun, P. Pd@MoS2 nanosheets based room-temperature hydrogen sensor. Mater. Lett. 2025, 390, 138458. [Google Scholar]
- Yang, Y.; He, Y.; Hu, S.; Li, Z. Self-Embedded Schottky Junctions in Liquid-Metal-Derived 2D Oxides for Fast and Selective Room-Temperature H2 Sensing. Adv. Funct. Mater. 2025, 35, 2500605. [Google Scholar] [CrossRef]
- Saadh, M.J.; Basem, A.; Makasana, J.; Sharma, P. Electrical and work function-based chemical gas sensors utilizing NC3 and graphene combination. J. Phys. Chem. Solids 2025, 197, 112443. [Google Scholar] [CrossRef]

| Materials | Tem. (°C) | H2 Con. (ppm) | Response | LOD c | Ref. |
|---|---|---|---|---|---|
| 3D Pt/SnO2 nanotubes | RT | 100 | 16.5% a | 285 ppb | This work |
| CeO2-doped SnO2 | 160 | 50 | 23.7 b | 10 ppm | [23] |
| Pd-doped rGO/ZnO–SnO2 | 380 | 100 | 9.4 b | 50 ppb | [24] |
| Ce-doped SLG/SnO2 | 250 | 10 | 2.49 b | 500 ppb | [25] |
| Pd@MoS2 | RT d | 15,000 | 4.44 b | 300 ppm | [26] |
| In-In2O3 | RT d | 1500 | 3.4 b | / | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Tian, Y.; Kang, Y.; Yan, J. Development of a Three-Dimensional Nanostructure SnO2-Based Gas Sensor for Room-Temperature Hydrogen Detection. Sensors 2025, 25, 4784. https://doi.org/10.3390/s25154784
Song Z, Tian Y, Kang Y, Yan J. Development of a Three-Dimensional Nanostructure SnO2-Based Gas Sensor for Room-Temperature Hydrogen Detection. Sensors. 2025; 25(15):4784. https://doi.org/10.3390/s25154784
Chicago/Turabian StyleSong, Zhilong, Yi Tian, Yue Kang, and Jia Yan. 2025. "Development of a Three-Dimensional Nanostructure SnO2-Based Gas Sensor for Room-Temperature Hydrogen Detection" Sensors 25, no. 15: 4784. https://doi.org/10.3390/s25154784
APA StyleSong, Z., Tian, Y., Kang, Y., & Yan, J. (2025). Development of a Three-Dimensional Nanostructure SnO2-Based Gas Sensor for Room-Temperature Hydrogen Detection. Sensors, 25(15), 4784. https://doi.org/10.3390/s25154784









