Consistency Is Key: A Secondary Analysis of Wearable Motion Sensor Accuracy Measuring Knee Angles Across Activities of Daily Living Before and After Knee Arthroplasty
Abstract
Highlights
- Wearable motion sensors accurately measure knee angles before/after total knee arthroplasty (TKA).
- Error computing knee angles was equivalent regardless of activity completed.
- Wearable motion sensors can be reliably deployed before/after TKA.
- Future studies can leverage this technology to quantify preoperative/postoperative function.
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrollment and Participants
2.2. Study Flow (Figure 2)
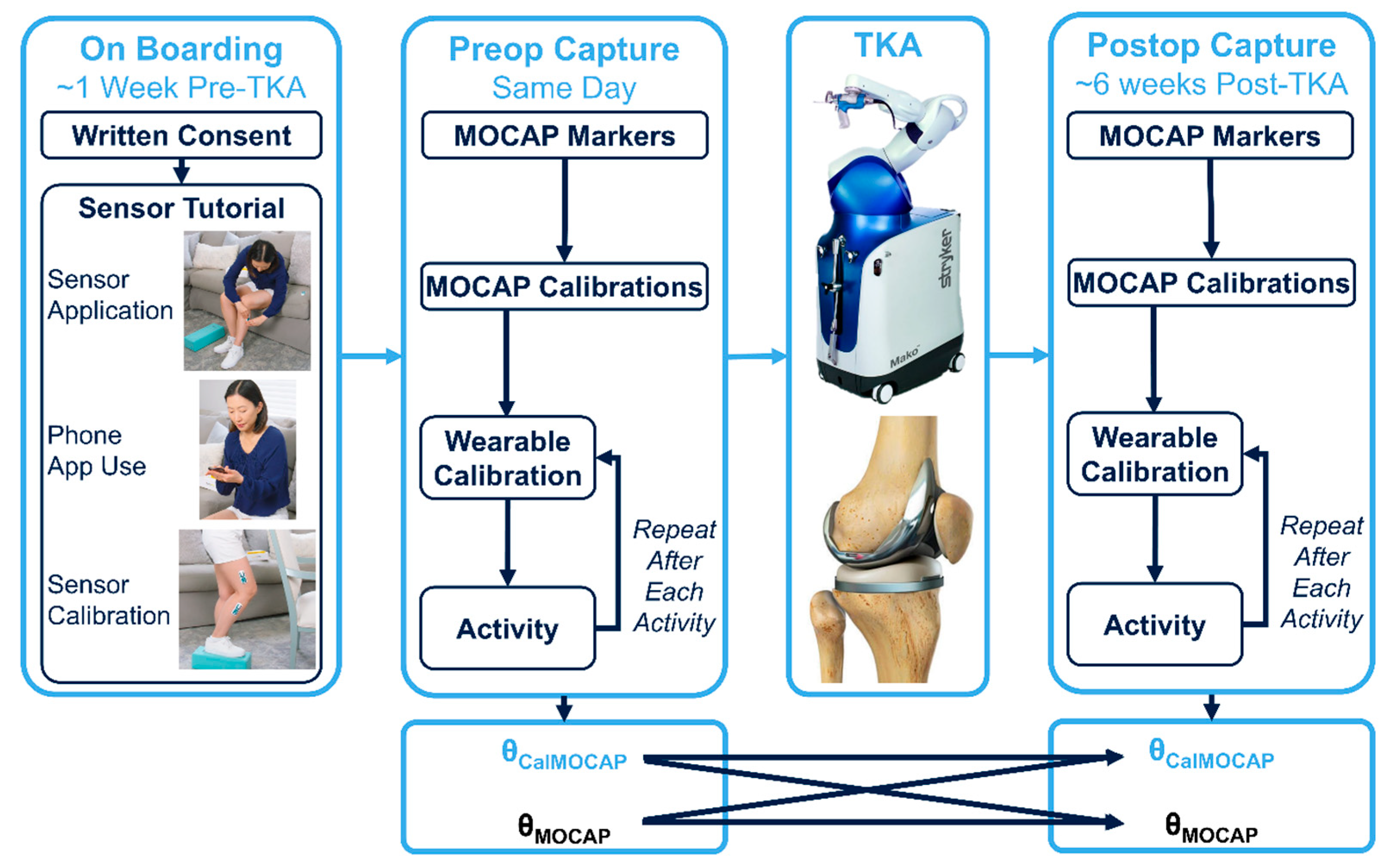
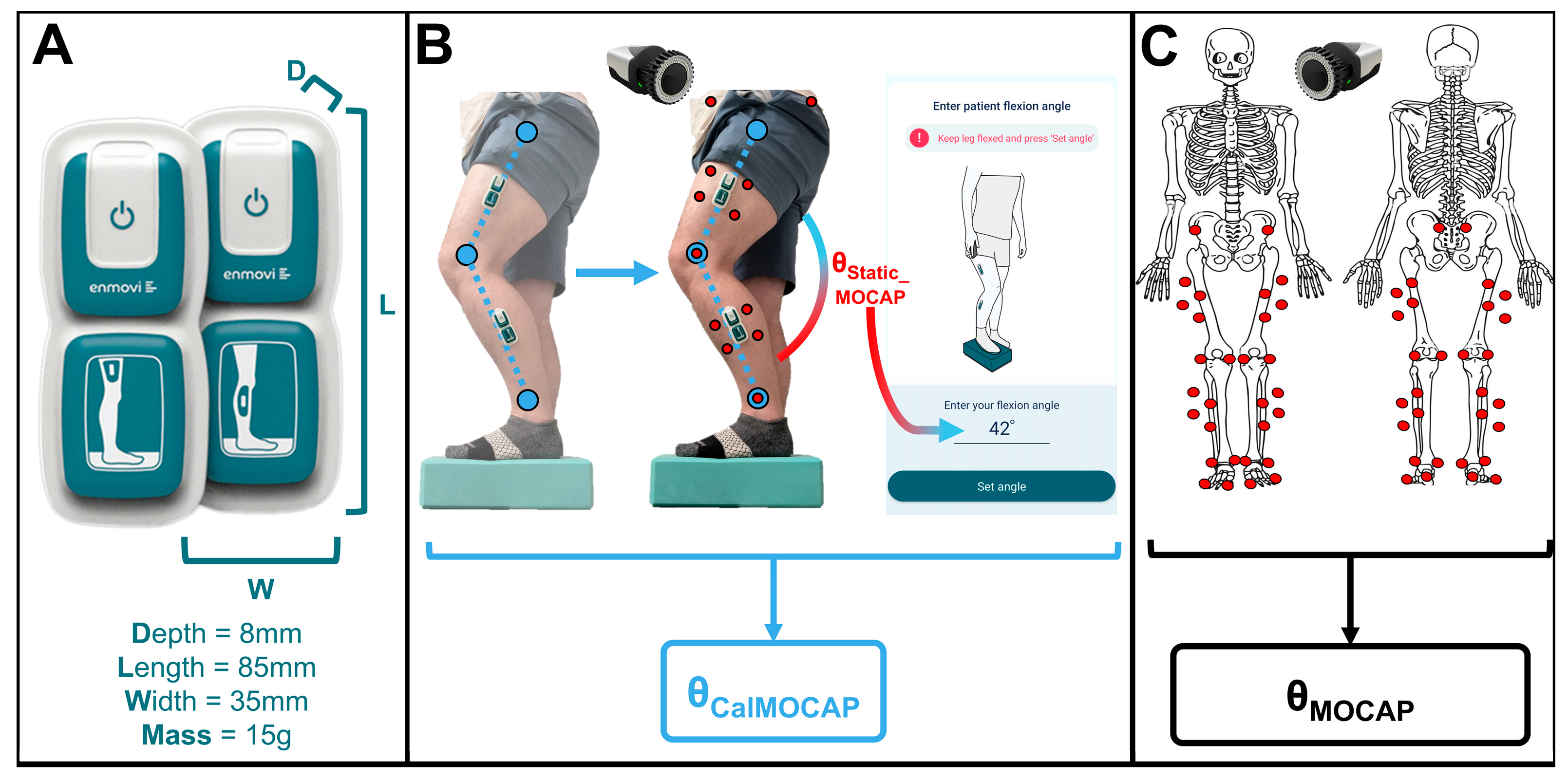
2.2.1. Biomechanics Instrumentation
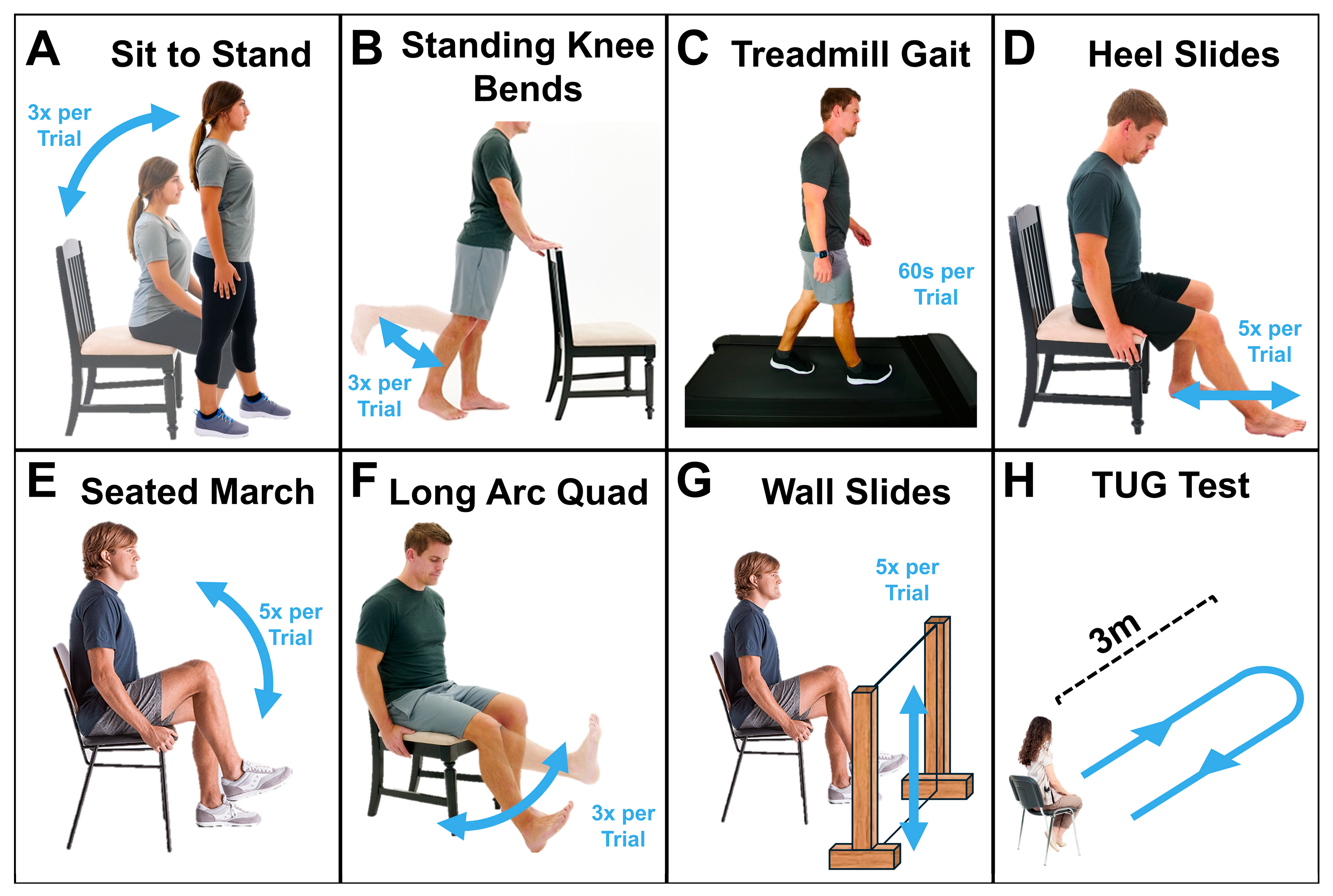
2.2.2. Activities (Figure 3)
2.3. Data Analyses
2.3.1. QTM and V3D Processing
2.3.2. Wearable Sensor Processing
2.3.3. Statistics
3. Results
3.1. Participant Characteristics
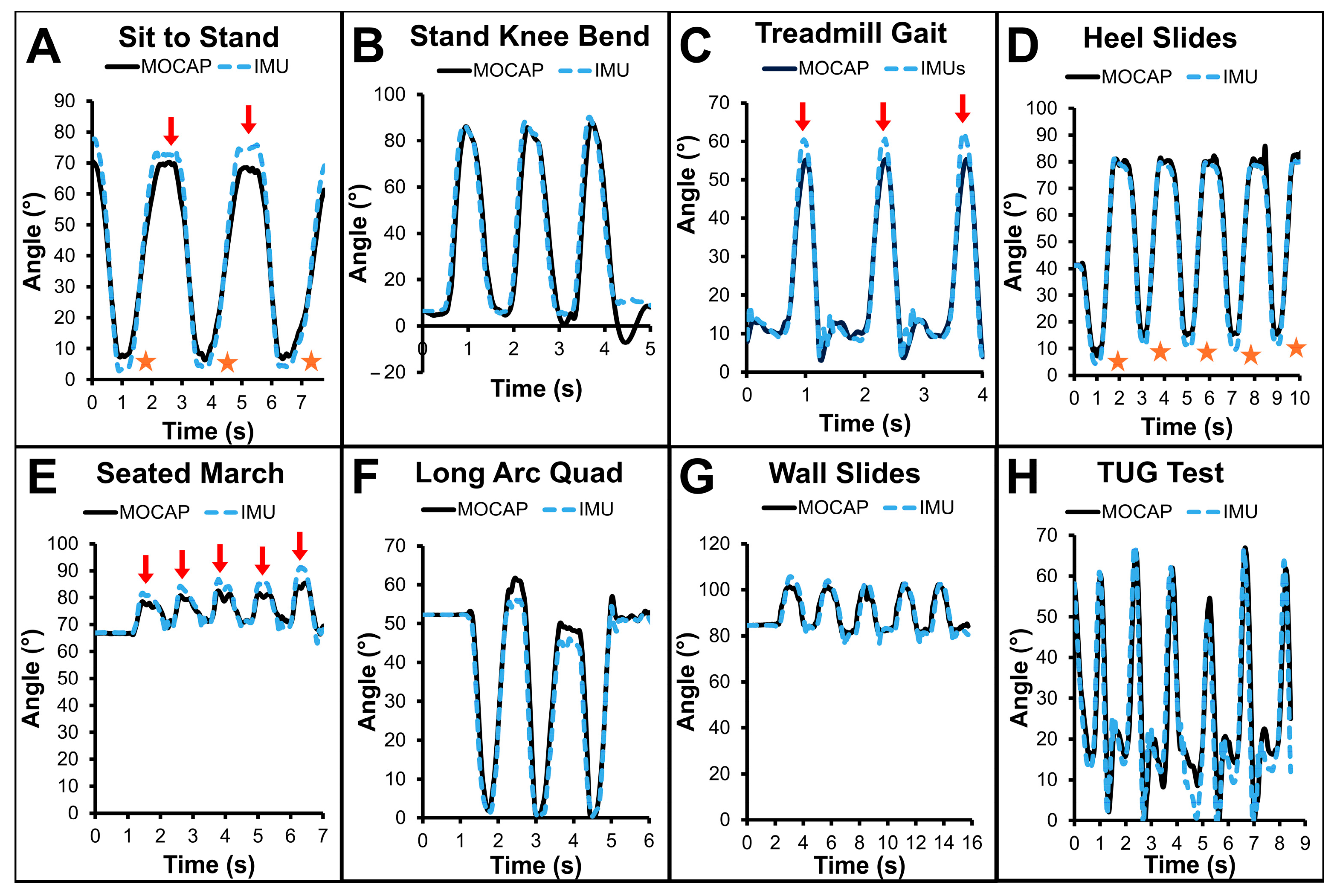
3.2. Example Data (Figure 4)
3.3. Results—Cohort Error Metrics
4. Discussion
4.1. Summary
4.2. Example Subject Data (Figure 4)
4.3. Discussion—Cohort Error Metrics
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROM | Range of motion |
| TKA | Total knee arthroplasty |
| MOCAP | Motion capture |
| NJ | New Jersey |
| IMU | Inertial measurement unit |
| ANOVA | Analysis of variance |
| OA | Osteoarthritis |
| ADL | Activity of daily living |
| IRB | Institutional review board |
| BMI | Body mass index |
| FL | Florida |
| CR | Cruciate retaining |
| QTM | Qualisys track manager |
| V3D | Visual3D |
| DOF | Degree of freedom |
| 3D | Three dimensions |
| AIM | Automatic identification of markers |
| TUG | Timed up and go |
| S2S | Sit to stand |
| SKB | Standing knee bends |
| HS | Heel slides |
| SM | Seated march |
| LAQ | Long arc quadriceps exercise |
| WS | Wall slides |
References
- Choi, Y.-J.; Ra, H.J. Patient Satisfaction after Total Knee Arthroplasty. Knee Surg. Relat. Res. 2016, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, L.; Pumilia, C.A.; Sarpong, N.O.; Martin, G. Patient Satisfaction, Functional Outcomes, and Implant Survivorship in Patients Undergoing Customized Cruciate-Retaining TKA. JBJS Rev. 2021, 9, e20.00074-7. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D.J. Patient Satisfaction after Total Knee Arthroplasty: Who Is Satisfied and Who Is Not? Clin. Orthop. Relat. Res. 2010, 468, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dolan, P.; Shaw, R.; Tsuchiya, A.; Williams, A. QALY Maximisation and People’s Preferences: A Methodological Review of the Literature. Health Econ. 2005, 14, 197–208. [Google Scholar] [CrossRef]
- Losina, E.; Walensky, R.P.; Kessler, C.L.; Emrani, P.S.; Reichmann, W.M.; Wright, E.A.; Holt, H.L.; Solomon, D.H.; Yelin, E.; Paltiel, A.D.; et al. Cost-Effectiveness of Total Knee Arthroplasty in the United States: Patient Risk and Hospital Volume. Arch. Intern. Med. 2009, 169, 1113. [Google Scholar] [CrossRef]
- Neumann, P.J.; Cohen, J.T.; Weinstein, M.C. Updating Cost-Effectiveness—The Curious Resilience of the $50,000-per-QALY Threshold. N. Engl. J. Med. 2014, 371, 796–797. [Google Scholar] [CrossRef]
- Noble, P.C.; Conditt, M.A.; Cook, K.F.; Mathis, K.B. The John Insall Award: Patient Expectations Affect Satisfaction with Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2006, 452, 35–43. [Google Scholar] [CrossRef]
- Robertsson, O.; Dunbar, M.; Pehrsson, T.; Knutson, K.; Lidgren, L. Patient Satisfaction after Knee Arthroplasty: A Report on 27,372 Knees Operated on between 1981 and 1995 in Sweden. Acta Orthop. Scand. 2000, 71, 262–267. [Google Scholar] [CrossRef]
- Kubo, M.; Maeda, T.; Kumagai, K.; Amano, Y.; Kawasaki, T.; Imai, S. Good Postoperative Flexion Angle Improves Knee Function and Improvement of Flexion Angle Increases Patient Satisfaction After Total Knee Arthroplasty. J. Arthroplast. 2021, 36, 3137–3140. [Google Scholar] [CrossRef]
- Carter, T.I.; Pansy, B.; Wolff, A.L.; Hillstrom, H.J.; Backus, S.I.; Lenhoff, M.; Wolfe, S.W. Accuracy and Reliability of Three Different Techniques for Manual Goniometry for Wrist Motion: A Cadaveric Study. J. Hand Surg. 2009, 34, 1422–1428. [Google Scholar] [CrossRef]
- Gajdosik, R.L.; Bohannon, R.W. Clinical Measurement of Range of Motion. Phys. Ther. 1987, 67, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.E.; Hepworth, T.; Wembridge, K. Accuracy and Reliability of Knee Goniometry Methods. J. Exp. Orthop. 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.L.; Christensen, M.; Christensen, S.S.; Olsen, M.; Bandholm, T. Reliability of Knee Joint Range of Motion and Circumference Measurements after Total Knee Arthroplasty: Does Tester Experience Matter? Physiother. Res. Int. 2010, 15, 126–134. [Google Scholar] [CrossRef]
- Ore, V.; Nasic, S.; Riad, J. Lower Extremity Range of Motion and Alignment: A Reliability and Concurrent Validity Study of Goniometric and Three-Dimensional Motion Analysis Measurement. Heliyon 2020, 6, e04713. [Google Scholar] [CrossRef]
- Peters, P.G.; Herbenick, M.A.; Anloague, P.A.; Markert, R.J.; Rubino, J.L. Knee Range of Motion: Reliability and Agreement of 3 Measurement Methods. Phys. Ther. Fac. Publ. 2011, 32, E249–E252. [Google Scholar]
- Bolink, S.A.A.N.; Grimm, B.; Heyligers, I.C. Patient-Reported Outcome Measures versus Inertial Performance-Based Outcome Measures: A Prospective Study in Patients Undergoing Primary Total Knee Arthroplasty. Knee 2015, 22, 618–623. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Lin, C.-Y.; Kuo, Y.-J.; Lee, O.K.-S. Feasibility and Effect of a Wearable Motion Sensor Device in Facilitating In-Home Rehabilitation Program in Patients after Total Knee Arthroplasty: A Preliminary Study. Appl. Sci. 2022, 12, 2433. [Google Scholar] [CrossRef]
- Kontadakis, G.; Chasiouras, D.; Proimaki, D.; Halkiadakis, M.; Fyntikaki, M.; Mania, K. Gamified Platform for Rehabilitation after Total Knee Replacement Surgery Employing Low Cost and Portable Inertial Measurement Sensor Node. Multimedia Tools Appl. 2020, 79, 3161–3188. [Google Scholar] [CrossRef]
- Chapman, R.M.; Moschetti, W.E.; Van Citters, D.W. Stance and Swing Phase Knee Flexion Recover at Different Rates Following Total Knee Arthroplasty: An Inertial Measurement Unit Study. J. Biomech. 2019, 84, 129–137. [Google Scholar] [CrossRef]
- Chapman, R.M.; Moschetti, W.E.; Van Citters, D.W. Is Clinically Measured Knee Range of Motion after Total Knee Arthroplasty ‘Good Enough?’: A Feasibility Study Using Wearable Inertial Measurement Units to Compare Knee Range of Motion Captured during Physical Therapy versus at Home. Med. Nov. Technol. Devices 2021, 11, 100085. [Google Scholar] [CrossRef]
- Franco, T.; Sestrem, L.; Henriques, P.R.; Alves, P.; Varanda Pereira, M.J.; Brandão, D.; Leitão, P.; Silva, A. Motion Sensors for Knee Angle Recognition in Muscle Rehabilitation Solutions. Sensors 2022, 22, 7605. [Google Scholar] [CrossRef]
- Nissa, R.; Karmakar, N.C.; Baghini, M.S. A Wearable Accelerometer-Based System for Knee Angle Monitoring During Physiotherapy. IEEE Sens. J. 2024, 24, 21417–21425. [Google Scholar] [CrossRef]
- Fan, B.; Xia, H.; Xu, J.; Li, Q.; Shull, P.B. IMU-Based Knee Flexion, Abduction and Internal Rotation Estimation during Drop Landing and Cutting Tasks. J. Biomech. 2021, 124, 110549. [Google Scholar] [CrossRef]
- Antunes, R.; Jacob, P.; Meyer, A.; Conditt, M.A.; Roche, M.W.; Verstraete, M.A. Accuracy of Measuring Knee Flexion after TKA through Wearable IMU Sensors. J. Funct. Morphol. Kinesiol. 2021, 6, 60. [Google Scholar] [CrossRef]
- Chapman, R.M.; Taylor, K.B.; Kaczynski, E.; Khodabakhsh, S.; Richards, S.; Hutchinson, J.B.; Marchand, R.C. Accuracy amidst Errors: Evaluating a Commercially Available Wearable Sensor System and Its Associated Calibration Procedures for Monitoring Sagittal Knee Motion in Patients Undergoing Total Knee Arthroplasty. Knee 2025, 54, 316–328. [Google Scholar] [CrossRef]
- Forsyth, L.; Ligeti, A.; Blyth, M.; Clarke, J.V.; Riches, P.E. Validity of Wearable Sensors for Total Knee Arthroplasty (TKA) Rehabilitation: A Study in Younger and Older Healthy Participants. Knee 2024, 51, 292–302. [Google Scholar] [CrossRef]
- Mistry, J.; Elmallah, R.; Bhave, A.; Chughtai, M.; Cherian, J.; McGinn, T.; Harwin, S.; Mont, M. Rehabilitative Guidelines after Total Knee Arthroplasty: A Review. J. Knee Surg. 2016, 29, 201–217. [Google Scholar] [CrossRef]
- Dávila Castrodad, I.M.; Recai, T.M.; Abraham, M.M.; Etcheson, J.I.; Mohamed, N.S.; Edalatpour, A.; Delanois, R.E. Rehabilitation Protocols Following Total Knee Arthroplasty: A Review of Study Designs and Outcome Measures. Ann. Transl. Med. 2019, 7, S255. [Google Scholar] [CrossRef]
- Artz, N.; Elvers, K.T.; Lowe, C.M.; Sackley, C.; Jepson, P.; Beswick, A.D. Effectiveness of Physiotherapy Exercise Following Total Knee Replacement: Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2015, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Bączkowicz, D.; Skiba, G.; Czerner, M.; Majorczyk, E. Gait and Functional Status Analysis before and after Total Knee Arthroplasty. Knee 2018, 25, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.Z.; Greene, K.A.; Davis, R.S.; Kovacik, M.W.; Noe, D.A.; Askew, M.J. Measuring Flexion in Knee Arthroplasty Patients. J. Arthroplast. 2004, 19, 369–372. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.; Fineman, R.; Stirling, L. An Auto-Calibrating Knee Flexion-Extension Axis Estimator Using Principal Component Analysis with Inertial Sensors. Sensors 2018, 18, 1882. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C. Color Atlas of Skeletal Landmark Definitions: Guidelines for Reproducible Manual and Virtual Palpations; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ferber, R.; McClay Davis, I.; Williams Iii, D.S. Gender Differences in Lower Extremity Mechanics during Running. Clin. Biomech. 2003, 18, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Cappozzo, A.; Cappello, A.; Della Croce, U.; Pensalfini, F. Surface-Marker Cluster Design Criteria for 3-D Bone Movement Reconstruction. IEEE Trans. Biomed. Eng. 1997, 44, 1165–1174. [Google Scholar] [CrossRef]
- Kertis, J.D.; Fritz, J.M.; Long, J.T.; Harris, G.F. Static and Dynamic Calibration of an Eight-Camera Optical System for Human Motion Analysis. Crit. Rev. Phys. Rehabil. Med. 2010, 22, 49–59. [Google Scholar] [CrossRef]
- Grood, E.S.; Suntay, W.J. A Joint Coordinate System for the Clinical Description of Three-Dimensional Motions: Application to the Knee. ASME J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB Recommendation on Definitions of Joint Coordinate System of Various Joints for the Reporting of Human Joint Motion—Part I: Ankle, Hip, and Spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Madgwick, S.O.H.; Harrison, A.J.L.; Vaidyanathan, R. Estimation of IMU and MARG Orientation Using a Gradient Descent Algorithm. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1–7. [Google Scholar]
- Bell, A.L.; Pedersen, D.R.; Brand, R.A. A Comparison of the Accuracy of Several Hip Center Location Prediction Methods. J. Biomech. 1990, 23, 617–621. [Google Scholar] [CrossRef]
- Bell, A.L.; Brand, R.A.; Pedersen, D.R. Prediction of Hip Joint Centre Location from External Landmarks. Hum. Mov. Sci. 1989, 8, 3–16. [Google Scholar] [CrossRef]
- Jeon, T.H.; Lee, J.K. IMU-Based Joint Angle Estimation Under Various Walking and Running Conditions. J. Korean Soc. Precis. Eng. 2018, 35, 1199–1204. [Google Scholar] [CrossRef]
- Versteyhe, M.; De Vroey, H.; Debrouwere, F.; Hallez, H.; Claeys, K. A Novel Method to Estimate the Full Knee Joint Kinematics Using Low Cost IMU Sensors for Easy to Implement Low Cost Diagnostics. Sensors 2020, 20, 1683. [Google Scholar] [CrossRef] [PubMed]
- Rhudy, M.B.; Mahoney, J.M.; Altman-Singles, A.R. Knee Angle Estimation with Dynamic Calibration Using Inertial Measurement Units for Running. Sensors 2024, 24, 695. [Google Scholar] [CrossRef] [PubMed]
- Cornish, B.M.; Diamond, L.E.; Saxby, D.J.; Lloyd, D.G.; Shi, B.; Lyon, J.; Abbruzzese, K.; Gallie, P.; Maharaj, J. Sagittal Plane Knee Kinematics Can Be Measured during Activities of Daily Living Following Total Knee Arthroplasty with Two IMU. PLoS ONE 2024, 19, e0297899. [Google Scholar] [CrossRef]
- Malesevic, N.; Svensson, I.; Hägglund, G.; Antfolk, C. An Integrated Approach for Real-Time Monitoring of Knee Dynamics with IMUs and Multichannel EMG. Sensors 2023, 23, 8955. [Google Scholar] [CrossRef]
- Prill, R.; Walter, M.; Królikowska, A.; Becker, R. A Systematic Review of Diagnostic Accuracy and Clinical Applications of Wearable Movement Sensors for Knee Joint Rehabilitation. Sensors 2021, 21, 8221. [Google Scholar] [CrossRef] [PubMed]
- Fennema, M.C.; Bloomfield, R.A.; Lanting, B.A.; Birmingham, T.B.; Teeter, M.G. Repeatability of Measuring Knee Flexion Angles with Wearable Inertial Sensors. Knee 2019, 26, 97–105. [Google Scholar] [CrossRef]
- Chia, L.; Andersen, J.T.; McKay, M.J.; Sullivan, J.; Megalaa, T.; Pappas, E. Evaluating the Validity and Reliability of Inertial Measurement Units for Determining Knee and Trunk Kinematics during Athletic Landing and Cutting Movements. J. Electromyogr. Kinesiol. 2021, 60, 102589. [Google Scholar] [CrossRef]


| Error Metric | Test #1 | Test #2 | |||||
|---|---|---|---|---|---|---|---|
| p | Skewness | Kurtosis | Outliers | p | Skewness | Kurtosis | |
| Normally Distributed Data Thresholds | ≥0.05 | −1 < γ < 1 | −1 < κ < 1 | n = 0 | ≥0.05 | −1 < γ < 1 | −1 < κ < 1 |
| Average | <0.001 | −0.677 | 5.664 | n = 12 | 0.303 | 0.157 | 0.035 |
| Local Maxima | <0.001 | −0.807 | 4.605 | n = 7 | 0.643 | −0.084 | −0.012 |
| Local Minima | <0.001 | 0.473 | 1.300 | n = 8 | 0.363 | −0.099 | −0.162 |
| Activity | R2 | Slope | Intercept |
|---|---|---|---|
| Ideal | 1.0 | 1.00 | ±0.0° |
| Sit to Stand | 0.96 | 1.10 | −8.1° |
| Standing Knee Bend | 0.97 | 1.04 | −0.5° |
| Treadmill Gait | 0.86 | 0.98 | +1.8° |
| Heel Slides | 0.95 | 1.04 | −3.7° |
| Seated March | 0.93 | 1.02 | −2.4° |
| Long Arc Quad | 0.97 | 1.09 | −7.3° |
| Wall Slide | 0.92 | 1.02 | −1.4° |
| TUG Test | 0.93 | 1.06 | −4.6° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchand, R.C.; Taylor, K.B.; Kaczynski, E.C.; Richards, S.; Hutchinson, J.B.; Khodabakhsh, S.; Chapman, R.M. Consistency Is Key: A Secondary Analysis of Wearable Motion Sensor Accuracy Measuring Knee Angles Across Activities of Daily Living Before and After Knee Arthroplasty. Sensors 2025, 25, 3942. https://doi.org/10.3390/s25133942
Marchand RC, Taylor KB, Kaczynski EC, Richards S, Hutchinson JB, Khodabakhsh S, Chapman RM. Consistency Is Key: A Secondary Analysis of Wearable Motion Sensor Accuracy Measuring Knee Angles Across Activities of Daily Living Before and After Knee Arthroplasty. Sensors. 2025; 25(13):3942. https://doi.org/10.3390/s25133942
Chicago/Turabian StyleMarchand, Robert C., Kelly B. Taylor, Emily C. Kaczynski, Skye Richards, Jayson B. Hutchinson, Shayan Khodabakhsh, and Ryan M. Chapman. 2025. "Consistency Is Key: A Secondary Analysis of Wearable Motion Sensor Accuracy Measuring Knee Angles Across Activities of Daily Living Before and After Knee Arthroplasty" Sensors 25, no. 13: 3942. https://doi.org/10.3390/s25133942
APA StyleMarchand, R. C., Taylor, K. B., Kaczynski, E. C., Richards, S., Hutchinson, J. B., Khodabakhsh, S., & Chapman, R. M. (2025). Consistency Is Key: A Secondary Analysis of Wearable Motion Sensor Accuracy Measuring Knee Angles Across Activities of Daily Living Before and After Knee Arthroplasty. Sensors, 25(13), 3942. https://doi.org/10.3390/s25133942






