Prototype of a Textronic Sensor Created with a Physical Vacuum Deposition Process for Staphylococcus aureus Detection
Abstract
1. Introduction
2. Materials and Methods
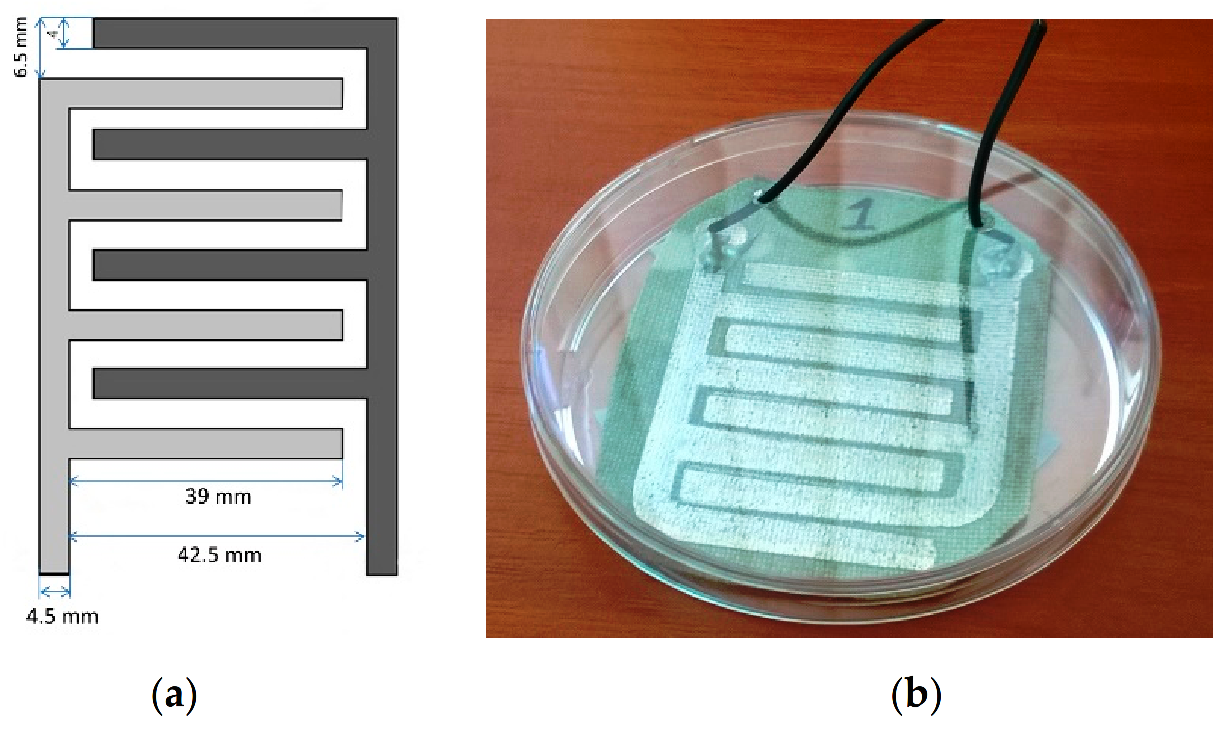
2.1. Sensor Preparation
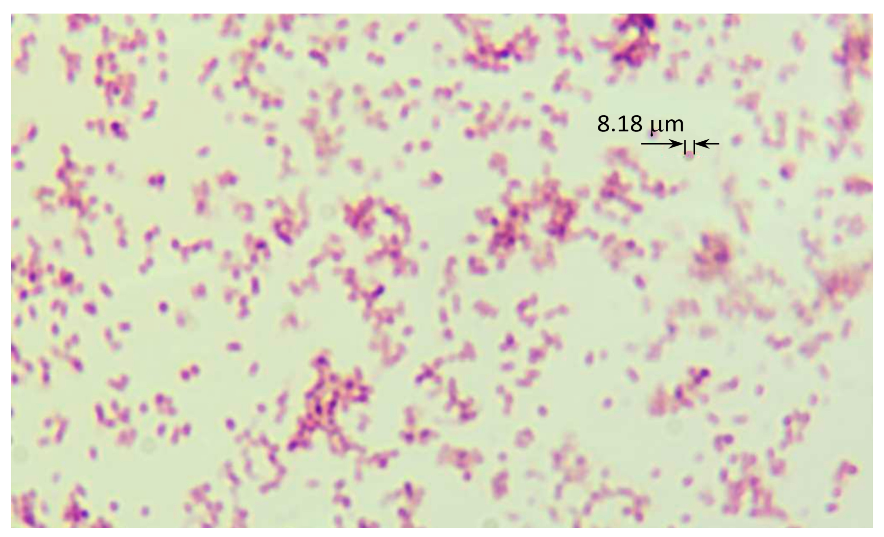
2.2. Bio Material
2.3. Measurement Method
2.4. Electrical Measurement
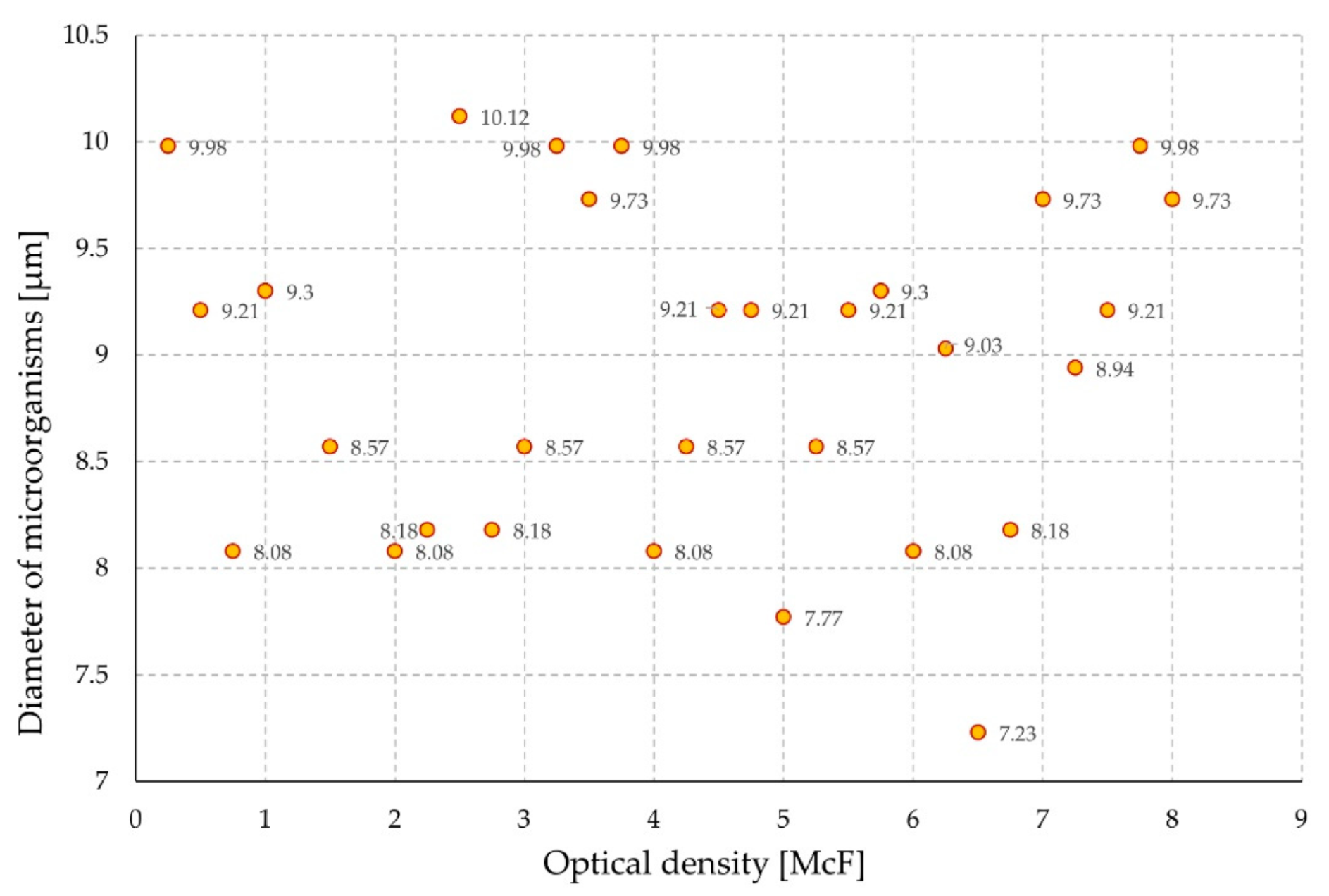
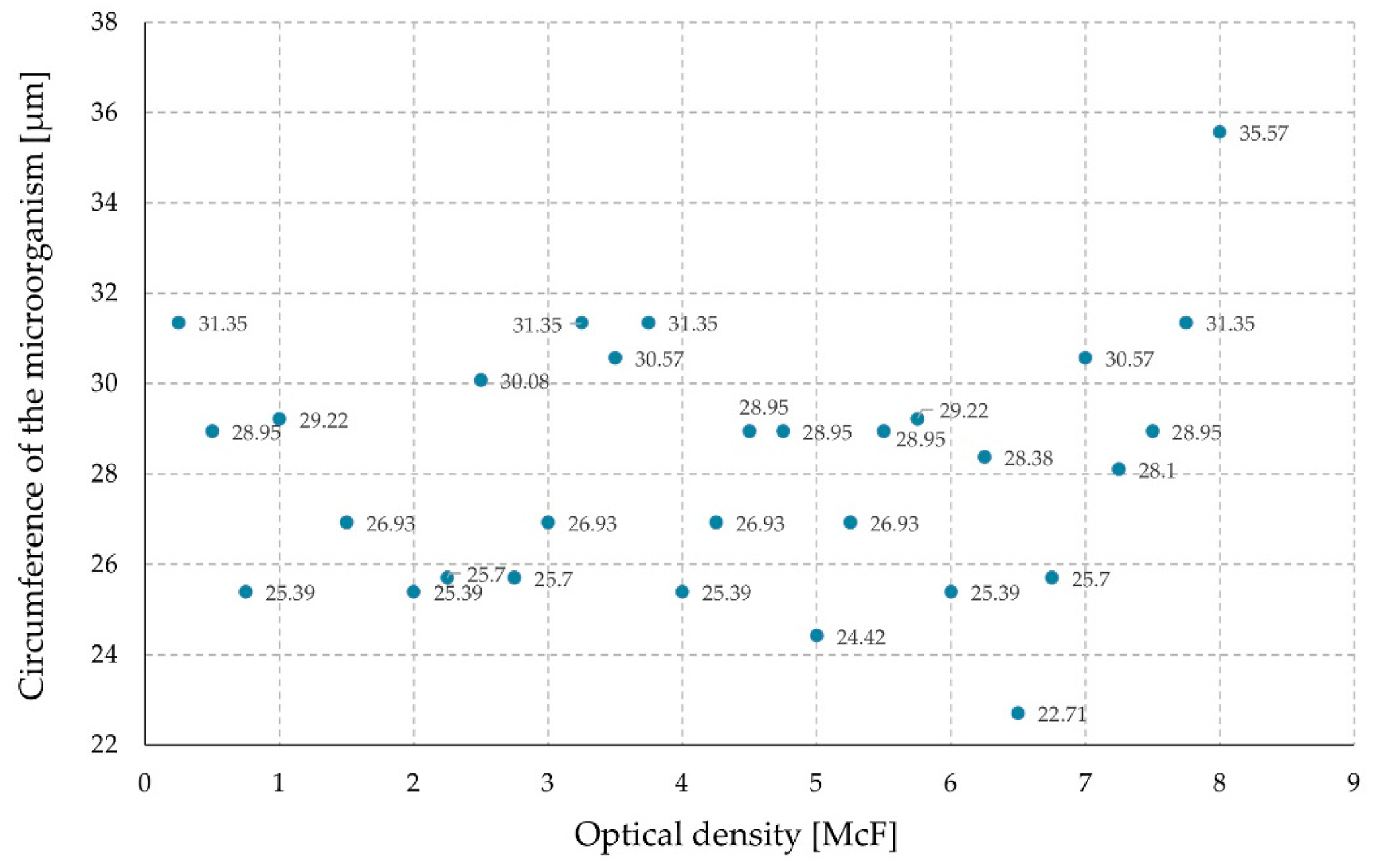
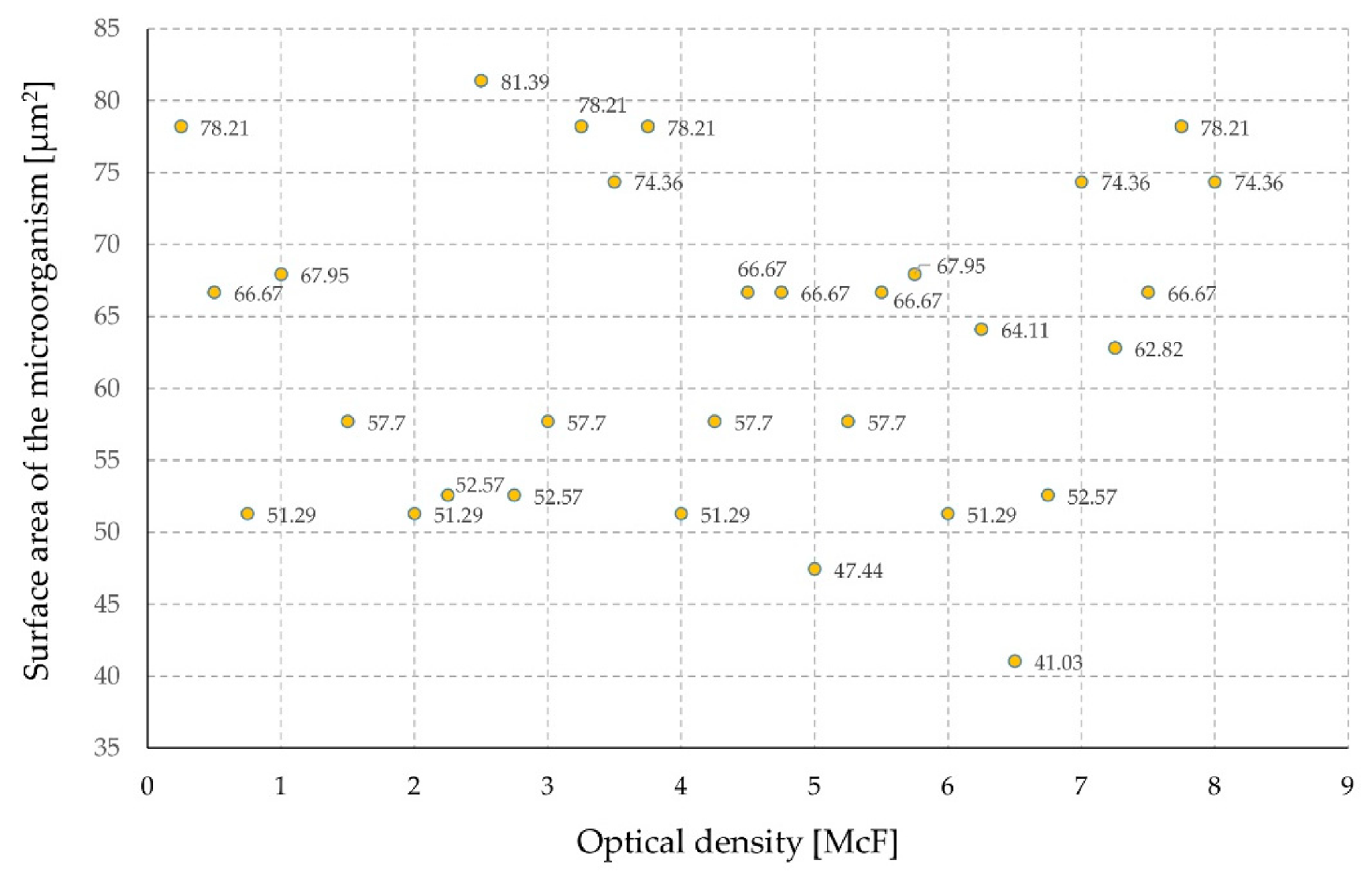
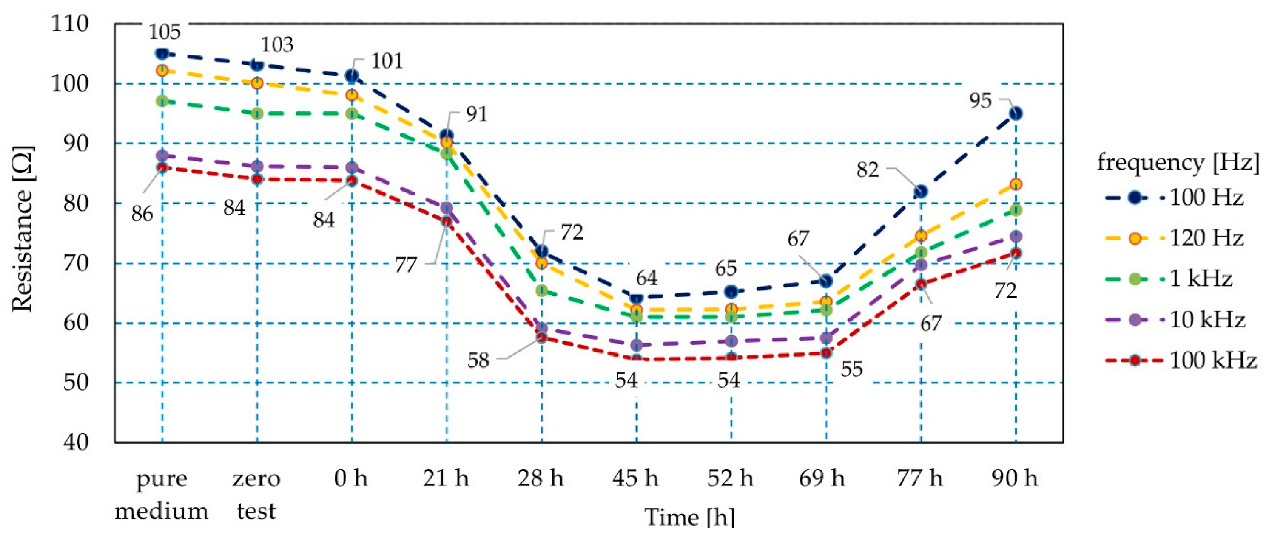
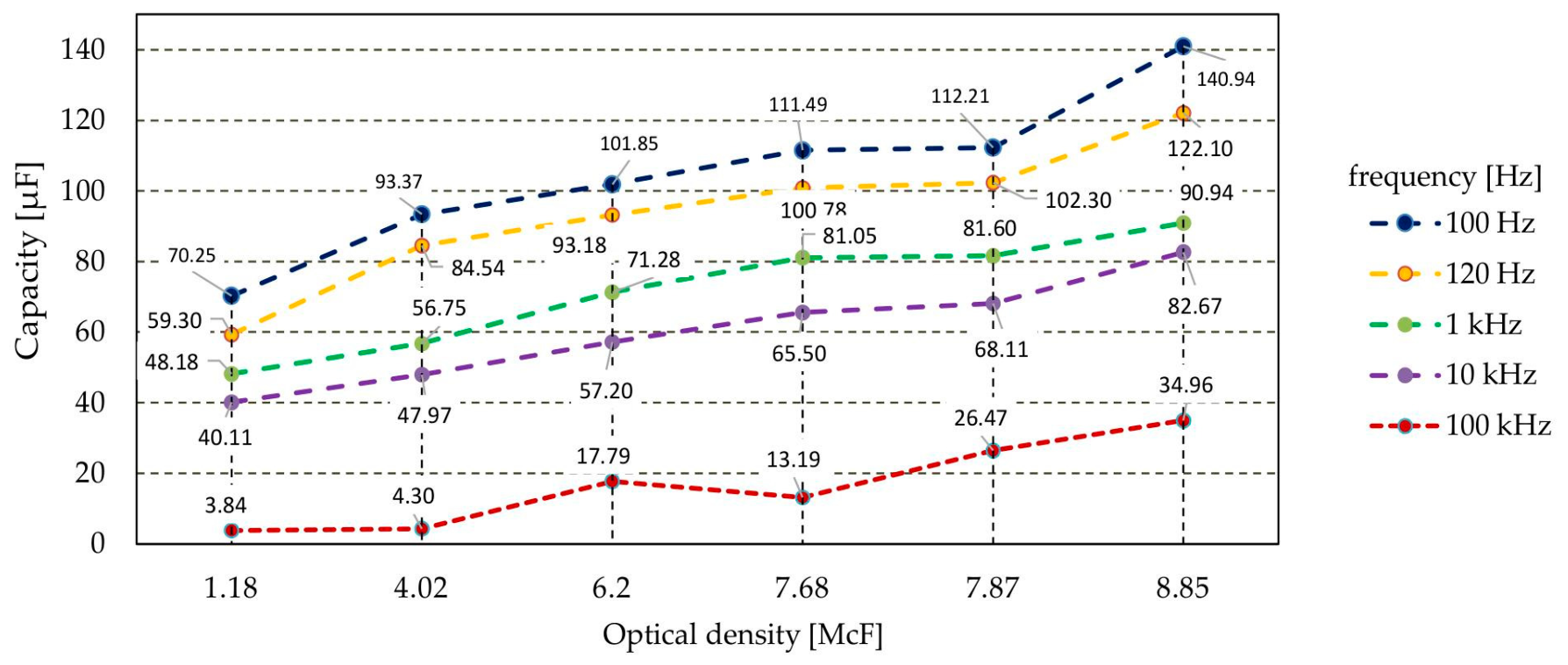
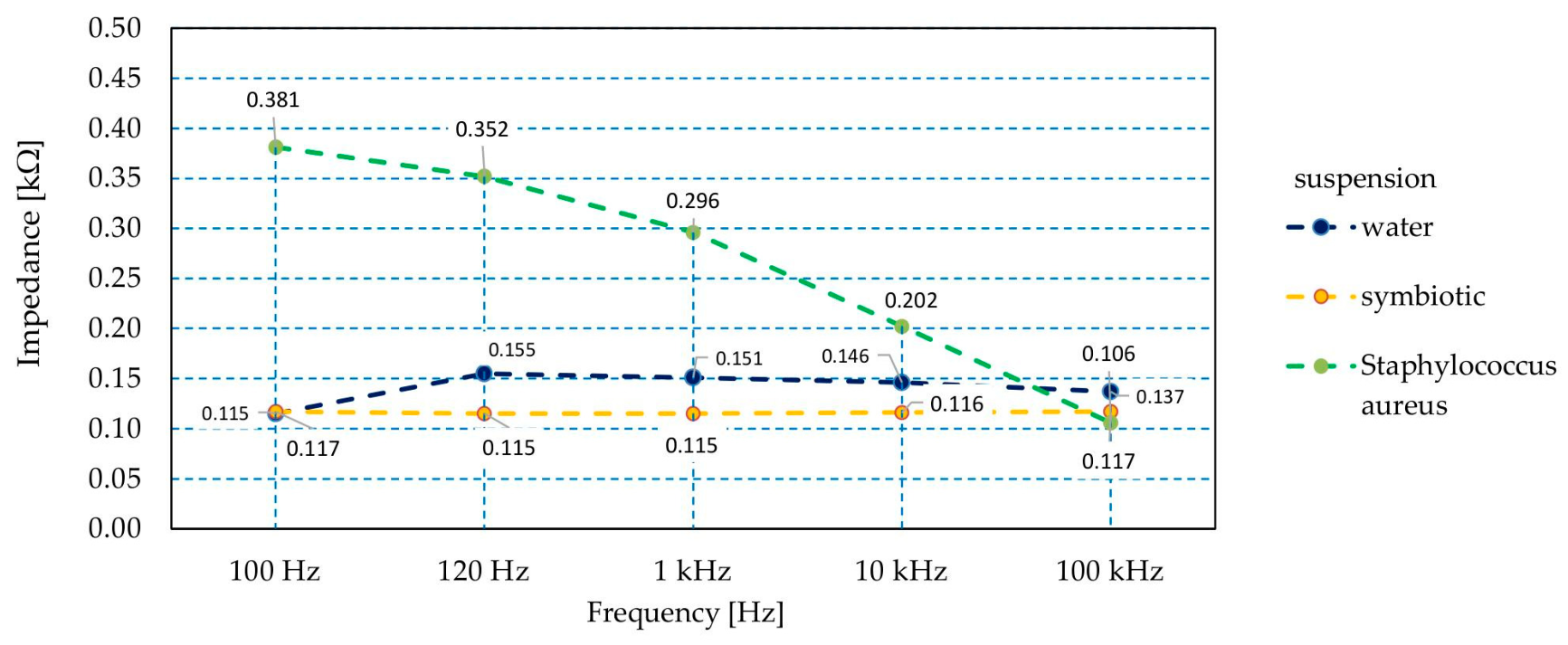
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malinowska, M.; Tokarz-Deptuła, B.; Deptuła, W. The human microbiome. Postępy Mikrobiol. 2017, 56, 33–42. [Google Scholar]
- Zych, M.A.; Górska, E.B.; Jankiewicz, U.; Kowalczyk, P. Disinfectants and their effectiveness on skin microorganisms. Nowa Med. 2013, 1, 31–34. [Google Scholar]
- Akiyama, H.; Morizane, S.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Assessment of Streptococcus pyogenes microcolony formation in infected skin by confocal laser scanning microscopy. J. Dermatol. Sci. 2003, 32, 193–199. [Google Scholar] [CrossRef]
- El Baze, P.; Thyss, A.; Caldani, C.; Juhlin, L.; Schneider, M.; Ortonne, J.P. Pseudomonas aeruginosa O-11 folliculitis. Development into ecthyma gangrenosum in immunosuppressed patients. Arch. Dermatol. 1985, 121, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Tseng, C.; Pei, Z.; Blaser, M.J. Molecular analysis of human forearm superficial skin bacterial biota. Proc. Natl. Acad. Sci. USA 2007, 104, 2927–2932. [Google Scholar] [CrossRef]
- Noble, W.C. Skin bacteriology and the role of Staphylococcus aureus in infection. Br. J. Dermatol. 1998, 139, 9–12. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Leyden, J.J.; McGinley, K.J.; Nordstrom, K.M.; Webster, G.F. Skin microbiota. J. Investig. Dermatol. 1987, 88, 65–72. [Google Scholar] [CrossRef]
- Zych, M.A.; Górska, E.B.; Jankiewicz, U.; Kowalczyk, P.; Stępień, W. Diseases caused by microorganisms on the skin. Med. Rodz. 2013, 4, 158–163. [Google Scholar]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.C.; Valkenburg, H.A.; Wolters, C.H.L. Carriage of Staphylococcus aureus in random samples of a normal population. J. Hyg. Camb. 1967, 65, 567–573. [Google Scholar] [CrossRef]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, Underlying mechanisms, and associated risk. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Gu, H.; Hao, L.; Ye, H.; Gong, W.; Wang, Z. A Review of the Methods for Detection of Staphylococcus aureus Enterotoxins. Toxins 2016, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Dobroczyńska, J.I. Detection Systems for Microbiological Identification. Available online: http://www.eko-dok.pl/2013/12.pdf (accessed on 16 October 2020).
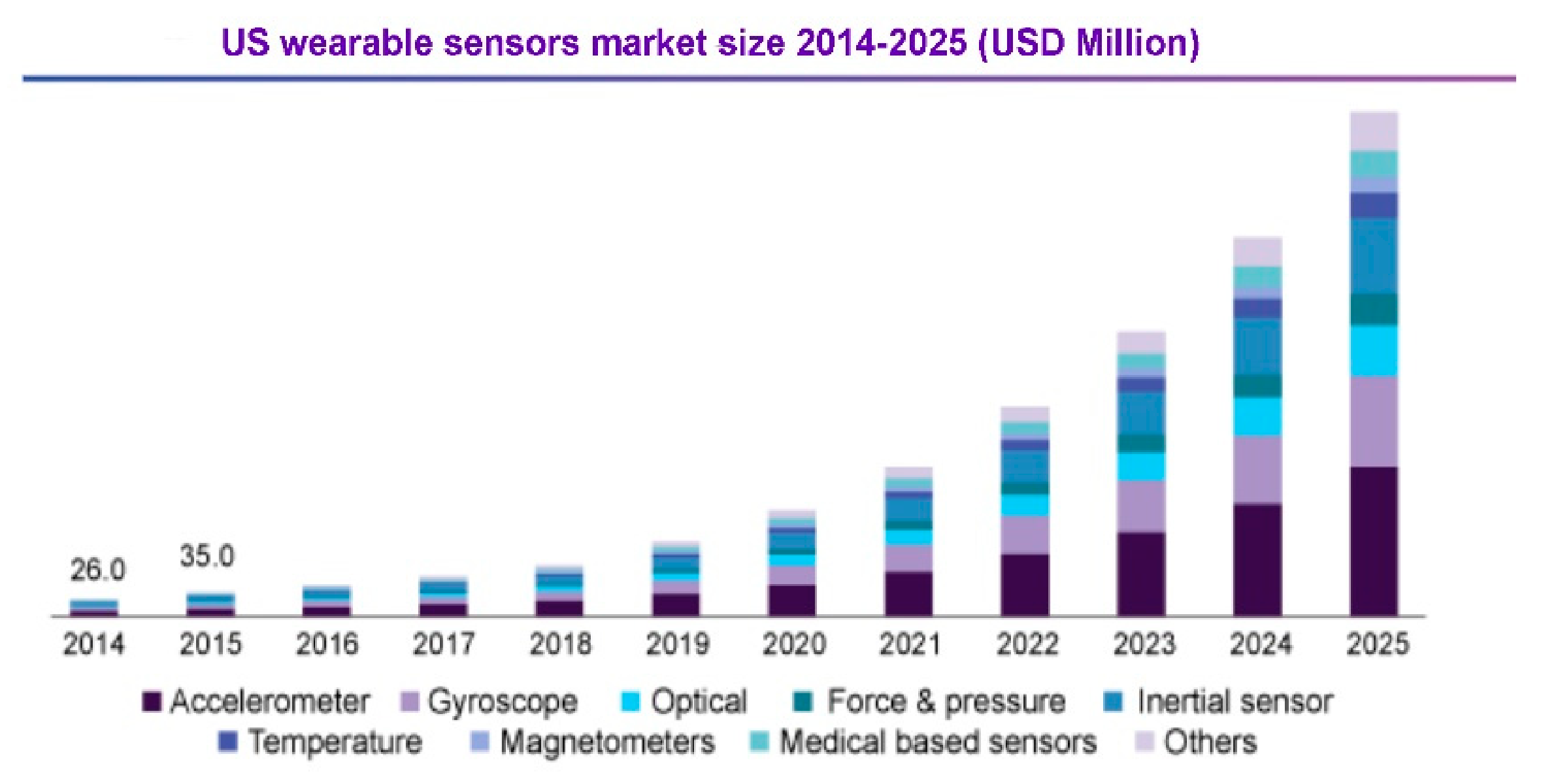
- Global Wearable Sensors Market Size, Industry Report, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/global-wearable-sensor-market (accessed on 12 September 2020).
- Rambausek, L.; Els Bruneel, E.; De Mey, G.; Van Langenhove, L. Polyimide Dielectric Layer on Filaments For Organic Field Effect Transistors: Choice Of Solvent, Solution Composition And Dip-Coating Speed. AUTEX Res. J. 2014, 14, 121–134. [Google Scholar] [CrossRef][Green Version]
- Andrew, T.L.; Zhang, L.; Cheng, N.; Baima, M.; Joon Kim, J.; Allison, L.; Hoxie, S. Melding Vapor-Phase Organic Chemistry and Textile Manufacturing To Produce Wearable Electronics. Acc. Chem. Res. 2018, 51, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Ziaja, J. ZnO thin film deposition with pulsed magnetron sputtering. Prz. Elektrotechniczny 2007, 83, 235–239. [Google Scholar]
- Tokarska, M.; Frydrysiak, M.; Zieba, J. Electrical properties of flat textile material as inhomegeneous and anisotropic structure. J. Mater. Sci. Mater. Electron. 2013, 24, 5061–5068. [Google Scholar] [CrossRef]
- Lada-Tondyra, E.; Jakubas, A. Modern Applications of Textronic Systems. Prz. Elektrotechniczny 2018, 94, 198–201. [Google Scholar]
- Stempien, Z.; Rybicki, E.; Rybicki, T.; Kozanecki, M. Reactive inkjet printing of PEDOT electroconductive layers on textile surfaces. Synth. Met. 2016, 217, 276–287. [Google Scholar] [CrossRef]
- Pawlak, R.; Lebioda, M.; Tomczyk, M.; Rymaszewski, J.; Korzeniewska, E.; Walczak, M. Modelling and applications of conductive elements on textile materials. COMPEL Int. J. Comput. Math. Electr. Electron. Eng. 2018, 37, 1645–1656. [Google Scholar] [CrossRef]
- Nazmul Islam, G.M.; Ali, A.; Collie, S. Textile sensors for wearable applications: A comprehensive review. Cellulose 2020, 27, 6103–6131. [Google Scholar] [CrossRef]
- Brennan, D.; Galvin, P. Flexible substrate sensors for multiplex biomarker monitoring. MRS Commun. 2018, 8, 627–641. [Google Scholar] [CrossRef]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, E.; Szczesny, A. Parasitic parameters of thin film structures created on flexible substrates in PVD process. Microelectron. Eng. 2018, 193, 62–64. [Google Scholar] [CrossRef]
- Madianos, L.; Skotadis, E.; Tsekenis, G.; Patsiouras, L.; Tsigkourakos, M.; Tsoukalas, D. Impedimetric nanoparticle aptasensor for selective and label free pesticide detection. Microelectron. Eng. 2018, 189, 39–45. [Google Scholar] [CrossRef]
- Oberländer, J.; Jildeh, Z.B.; Kirchner, P.; Wendeler, L.; Bromm, A.; Iken, H.; Wagner, P.; Keusgen, M.; Schöning, M.J. Study of Interdigitated Electrode Arrays Using Experiments and Finite Element Models for the Evaluation of Sterilization Processes. Sensors 2015, 15, 26115–26127. [Google Scholar] [CrossRef]
- Partel, S.; Kasemann, S.; Matylitskaya, V.; Thanner, C.; Dincer, C.; Urban, G. A simple fabrication process for disposable interdigitated electrode arrays with nanogaps for lab-on-a-chip applications. Microelectron. Eng. 2017, 173, 27–32. [Google Scholar] [CrossRef]
- Frydrysiak, M.; Korzeniewska, E.; Tęsiorowski, Ł. The textile resistive humidity sensor manufacturing via (PVD) sputtering method. Sens. Lett. 2015, 13, 998–1001. [Google Scholar] [CrossRef]
- Stempien, Z.; Kozicki, M.; Pawlak, R.; Korzeniewska, E.; Owczarek, G.; Poscik, A.; Sajna, D. Ammonia gas sensors ink-jet printed on textile substrates. In Proceedings of the IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Chiang, C.J.; Tsai, K.T.; Lee, Y.H.; Lin, H.W.; Yang, Y.L.; Shih, C.C.; Lin, C.Y.; Jeng, H.A.; Weng, Y.H.; Cheng, Y.Y.; et al. In situ fabrication of conducting polymer composite film as a chemical resistive CO2 gas sensor. Microelectron. Eng. 2013, 111, 409–415. [Google Scholar] [CrossRef]
- Tian, W.; Ho, Y.; Chen, C.; Kuo, C. Sensing performance of precisely ordered TiO2 nanowire gas sensors fabricated by electron-beam lithography. Sensors 2013, 13, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, N.S.; Ramli, M.M.; Abdullah, M.M.A.B.; Halin, D.S.C.; Isa, S.S.M.; Talip, L.F.A.; Danial, N.S.; Murad, S.A.Z. Interdigitated electrodes as impedance and capacitance biosensors: A review. AIP Conf. Proc. 2017, 1885, 020276. [Google Scholar] [CrossRef]
- Iswary, L.; Nazri, N.F.N.; Arshad, M.K.; Fathil, M.F.M.; Nuzaihan, M.; Subash, M.N.; Gopinath, C.B. Fabrication and characterization of aluminium interdigitated electrode hybrid with ZnO for cardiac troponin T biomarker detection. AIP Conf. Proc. 2018, 2045, 020038. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, X.; Zhang, Y.; Li, K.; Yan, C.; Wei, X.; Chen, L.; Zhen, H.; Zhou, H.; Zhang, S.; et al. Photoreactive and Metal-Platable Copolymer Inks for High-Throughput, Room-Temperature Printing of Flexible Metal Electrodes for Thin-Film Electronics. Adv. Mater. 2016. [Google Scholar] [CrossRef]
- Daud, A.I.; Khairul, W.M.; Isa, M.I.N.; Wahid, K.A.A. Study on Semiconductor Properties of Acetylide-Thiourea Fabricated onto Interdigitated Electrodes (IDEs) Platform towards Application in Gas Sensing Technology. Makara J. Technol. 2017, 21, 103–108. [Google Scholar] [CrossRef]
- Rymarczyk, T.; Nita, P.; Vejar, A.; Wos, M.; Stefaniak, B.; Adamkiewicz, P. Wareable mobile measuring device based on electrical tomography. Prz. Elektrotechniczny 2019, 95, 211–214. [Google Scholar] [CrossRef]
- Rymarczyk, T.; Klosowski, G.; Tchorzewski, P.; Cieplak, T.; Kozlowski, E. Area monitoring using the ERT method with multisensor electrodes. Prz. Elektrotechniczny 2019, 95, 153–156. [Google Scholar] [CrossRef]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef]
- Pawlak, R.; Lebioda, M. Electrical and thermal properties of heater-sensor microsystems patterned in TCO films for wide-range temperature applications from 15 K to 350 K. Sensors 2018, 18, 1831. [Google Scholar] [CrossRef]
- Lebioda, M.; Rymaszewski, J. Dynamic properties of cryogenic temperature sensors. Prz. Elektrotechniczny 2015, 91, 225–227. [Google Scholar] [CrossRef][Green Version]
- Scholza, J.; Nockea, G.; Hollstein, F.; Weissbach, A. Investigations on fabrics coated with precious metals using the magnetron sputter technique with regard to their anti-microbial properties. Surf. Coat. Technol. 2005, 192, 252–256. [Google Scholar] [CrossRef]
- Jun, L.Q.; bin Djaswadi, G.W.; bin Hawari, H.F.; Zakariya, M.A.B. Simulation of Interdigitated Electrodes (IDEs) Geometry Using COMSOL Multiphysics. In Proceedings of the International Conference on Intelligent and Advanced System, Kuala Lumpur, Malaysia, 13–14 August 2018. [Google Scholar] [CrossRef]
- Pawlak, R.; Tomczyk, M.; Walczak, M. The favorable and unfavorable effects of oxide and intermetallic phases in conductive materials using laser micro technologies. Mater. Sci. Eng. B Adv. Funct. Solid-State Mater. 2012, 177, 1273–1280. [Google Scholar] [CrossRef]




| Methodology | Recommended Reference Strains of Aerobic Bacteria |
|---|---|
| Infertility To test the fertility of substrates used and the suitability of the method | Staphylococcus aureus Bacillus subtilis Pseudomonas aeruginosa |
| To study the microbiological cleanness of non-vital products (quantitative microbiological qualities) | Staphylococcus aureus Bacillus subtilis Pseudomonas aeruginosa |
| To determine the effectiveness of protection against microbes (maintenance test) | Staphylococcus aureus Pseudomonas aeruginosa |
| To test the effectiveness of disinfectants | Staphylococcus aureus Pseudomonas aeruginosa Enterococcus hirae Escherichia coli |
| Ingredient | Value (g/L) |
|---|---|
| Pancreatin casein hydrolysate | 15 |
| Soy peptone | 5 |
| Sodium chloride | 5 |
| Agar | 15 |
| Ingredient | Value (g/L) |
|---|---|
| Peptone | 5 |
| Beef extract | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzeniewska, E.; Szczęsny, A.; Lipiński, P.; Dróżdż, T.; Kiełbasa, P.; Miernik, A. Prototype of a Textronic Sensor Created with a Physical Vacuum Deposition Process for Staphylococcus aureus Detection. Sensors 2021, 21, 183. https://doi.org/10.3390/s21010183
Korzeniewska E, Szczęsny A, Lipiński P, Dróżdż T, Kiełbasa P, Miernik A. Prototype of a Textronic Sensor Created with a Physical Vacuum Deposition Process for Staphylococcus aureus Detection. Sensors. 2021; 21(1):183. https://doi.org/10.3390/s21010183
Chicago/Turabian StyleKorzeniewska, Ewa, Artur Szczęsny, Piotr Lipiński, Tomasz Dróżdż, Paweł Kiełbasa, and Anna Miernik. 2021. "Prototype of a Textronic Sensor Created with a Physical Vacuum Deposition Process for Staphylococcus aureus Detection" Sensors 21, no. 1: 183. https://doi.org/10.3390/s21010183
APA StyleKorzeniewska, E., Szczęsny, A., Lipiński, P., Dróżdż, T., Kiełbasa, P., & Miernik, A. (2021). Prototype of a Textronic Sensor Created with a Physical Vacuum Deposition Process for Staphylococcus aureus Detection. Sensors, 21(1), 183. https://doi.org/10.3390/s21010183







