Demographic Differences in Behavior, Movement, and Habitat Use in the Toad-Headed Agama (Phrynocephalus versicolor) of the Gobi Desert (Dornogovi, Mongolia)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Between Demographic Class Comparisons
3.2. Within Demographic Class Comparisons
3.2.1. Adult Males
3.2.2. Adult Females
3.2.3. Juveniles
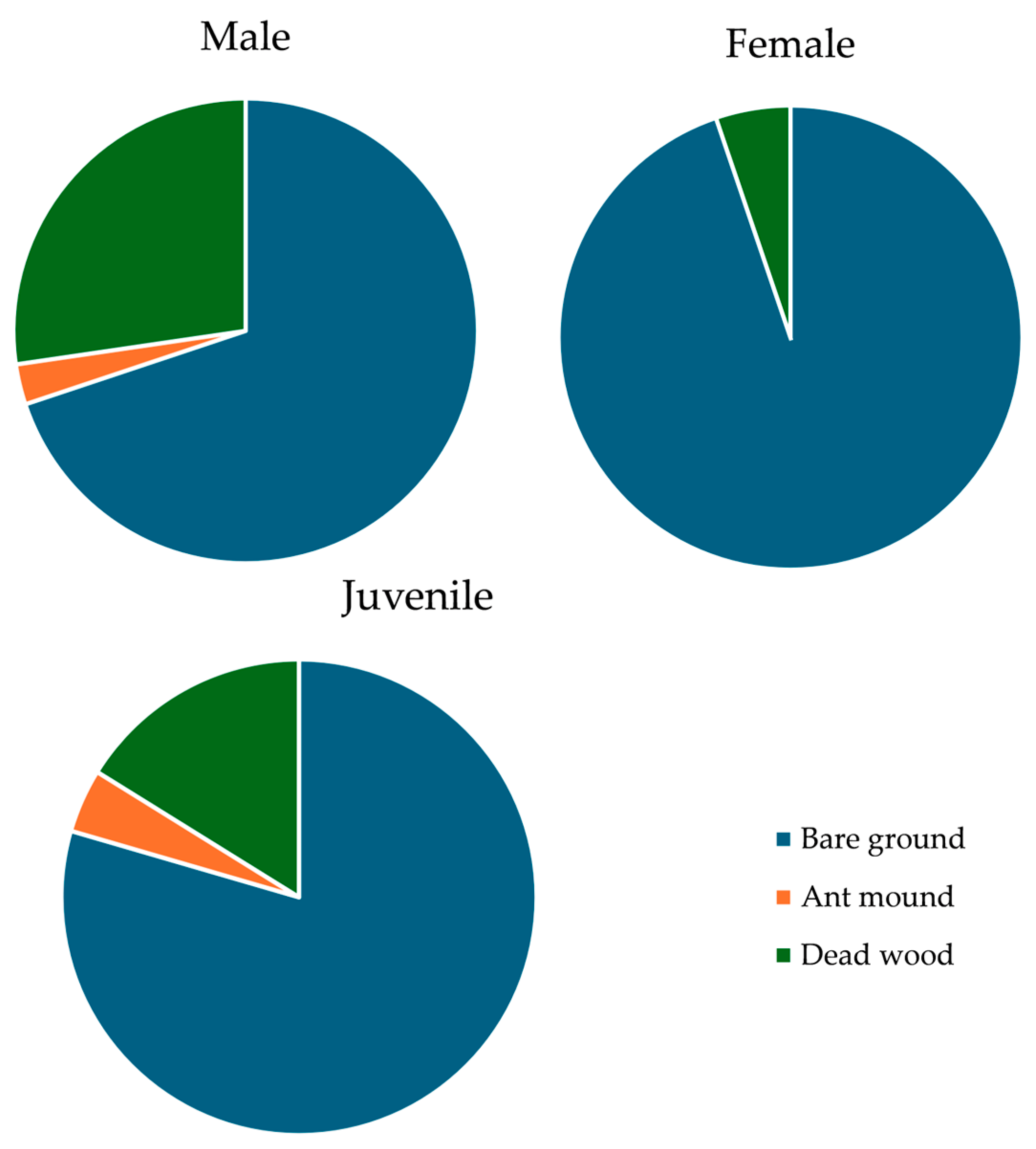
3.3. Habitat Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keren-Rotem, T.; Bouskila, A.; Geffen, E. Ontogenic habitat shift and risk of cannibalism in the common chameleon (Chamaeleo chamaeleon). Behav. Ecol. Sociobiol. 2006, 59, 723–731. [Google Scholar] [CrossRef]
- Paulissen, M.A. Ontogenetic and seasonal shifts in microhabitat use by the lizard Cnemidophorus sexlineatus. Copeia 1988, 1988, 1021–1029. [Google Scholar] [CrossRef]
- Stamps, J.A. The relationship between ontogenetic habitat shifts, competition and predator avoidance in a juvenile lizard (Anolis aeneus). Behav. Ecol. Sociobiol. 1983, 12, 19–33. [Google Scholar] [CrossRef]
- Herrel, A.; Gibb, A.C. Ontogeny of performance in vertebrates. Physiol. Biochem. Zool. 2006, 79, 1–6. [Google Scholar] [CrossRef]
- Landová, E.; Jančúchocá-Lásková, J.; Musilová, V.; Kadochová, S.; Frynta, D. Ontogenetic switch between alternative antipredatory strategies in the leopard gecko (Eublepharis macularius): Defensive threat versus escape. Behav. Ecol. Sociobiol. 2013, 67, 1113–1122. [Google Scholar] [CrossRef]
- Ruckstuhl, K.E.; Nuhaus, P. (Eds.) Sexual Segregation in Vertebrates: Ecology of the Two Sexes; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Hecht, L. The importance of considering age when quantifying wild animals’ welfare. Biol. Rev. 2021, 96, 2602–2616. [Google Scholar] [CrossRef] [PubMed]
- Carothers, J. Sexual selection and sexual dimorphism in some herbivorous lizards. Am. Nat. 1984, 124, 244–254. [Google Scholar] [CrossRef]
- Eifler, D.A.; Eifler, M.A. Escape tactics in the lizard Meroles cuneirostris. Amphibia-Reptilia 2014, 35, 383–389. [Google Scholar] [CrossRef]
- Kusaka, C.; Utsumi, K.L.; Staley, C.; Pedersen, R.; Valdivia, J.; Liu, E.; Caracalas, H.; Reynolds, H.; Eifler, M.A.; Eifler, D.A. Age dependent search behavior in the Colorado checkered whiptail (Aspidoscelis neotesselata). West. N. Am. Nat. 2021, 81, 518–528. [Google Scholar] [CrossRef]
- Diamond, K.; Olson, C.; Utsumi, K.L.; Eifler, M.A.; Eifler, D.A. Differences between juveniles and adults in habitat use, sprint performance, and morphology in the desert horned lizard, Phrynosoma platyrhinos. Ichthyol. Herpetol. 2024, 112, 347–352. [Google Scholar] [CrossRef]
- Cury de Barros, F.; Eduardo de Carvalho, J.; Abe, A.; Kohlsdorf, T. Fight versus flight: The interaction of temperature and body size determines antipredator behaviour in tegu lizards. Anim. Behav. 2010, 79, 83–88. [Google Scholar] [CrossRef]
- Martín, J.; López, P. Ontogenetic variation in antipredator behavior of Iberian rock lizards (Lacerta monticola): Effects of body-size-dependent thermal-exchange rates and costs of refuge use. Can. J. Zool. 2003, 81, 1131–1137. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, H.; Ma, L.; Ji, X. Differences in thermal preference and tolerance among three Phrynocephalus lizards (Agamidae) with different body sizes and habitat use. Asian Herpetol. Res. 2013, 4, 214–220. [Google Scholar] [CrossRef]
- Da Costa Siqueira, C.; Rocha, C.F.D. Predation by lizards as a mortality source for juvenile lizards in Brazil. S. Am. J. Herpetol. 2008, 3, 82–87. [Google Scholar] [CrossRef]
- Abrahms, B.; Seidel, D.P.; Dougherty, E.; Hazen, E.L.; Bograd, S.J.; Wilson, A.M.; McNutt, J.W.; Costa, D.P.; Blake, S.; Brashares, J.S.; et al. Suite of simple metrics reveals common movement syndromes across vertebrate taxa. Mov. Ecol. 2017, 5, 12. [Google Scholar] [CrossRef]
- Edelhoff, H.; Signer, J.; Balkenhol, N. Path segmentation for beginners: An overview of current methods for detecting changes in animal movement patterns. Mov. Ecol. 2016, 4, 21. [Google Scholar] [CrossRef]
- Eifler, D.A.; Eifler, M.A.; Orton, M.; Utsumi, K.L.; Jarray, M.; Zaidi, A.; Chammem, M. Movement and space use in three sympatric lacertid lizards (Acanthodactylus): Inter- and intraspecific comparisons. Afr. J. Ecol. 2023, 62, e13247. [Google Scholar] [CrossRef]
- McAlpine-Bellis, E.; Utsumi, K.; Diamond, K.; Klein, J.; Gilbert-Smith, S.; Garrison, G.; Eifler, M.; Eifler, D. Movement patterns and habitat use for the sympatric species Gambelia wislizenii and Aspidoscelis tigris. Ecol. Evol. 2023, 13, e10422. [Google Scholar] [CrossRef]
- Basso, E.; Ruiz, J.; Linscott, J.A.; Senner, N.R.; Weegman, M.; Ballard, B.; Navedo, J.G. Movement ecology during non-breeding season in a long-distance migratory shorebird: Are space use and movement patterns sex-biased? Behav. Ecol. Sociobiol. 2024, 78, 67. [Google Scholar] [CrossRef]
- Cain, S.; Solomon, T.; Leshem, Y.; Toledo, S.; Arnon, E.; Roulin, A.; Spiegel, O. Movement predictability of individual barn owls facilitates estimation of home range size and survival. Mov. Ecol. 2023, 11, 10. [Google Scholar] [CrossRef]
- Kays, R.; Hirsch, B.; Caillaud, D.; Mares, R.; Alavi, S.; Havmoller, R.W.; Crofoot, M. Multi-scale movement syndromes for comparative analyses of animal movement patterns. Mov. Ecol. 2023, 11, 61. [Google Scholar] [CrossRef]
- Masilkova, M.; Ciuti, S.; Podgorski, T.; Jezek, M.; Morelle, K.; Morera-Pujol, V. Consistent inter-individual variability in movement traits shapes the wild boar movement syndrome. Behav. Ecol. 2025, 36, araf036. [Google Scholar] [CrossRef]
- Moorcroft, P.R.; Lewis, M.A. Mechanistic Home Range Analysis; Monographs in Population Biology 43; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Smetzer, J.R.; Paxton, K.L.; Paxton, E.H. Individual and seasonal variation in the movement behavior of two tropical nectarivorous birds. Mov. Ecol. 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.L.; Morales, J.M.; Murray, D.; Fortin, M.-J. The effectiveness of Bayesian state-space models for estimating behavioural stats from movement paths. Methods Ecol. Evol. 2013, 4, 433–441. [Google Scholar] [CrossRef]
- Borah, B.; Beckman, N.G.G. Bird movement patterns in an agricultural landscape are mediated by both habitat conditions and traits. Biotropica 2023, 55, 1069–1080. [Google Scholar] [CrossRef]
- Hodel, F.H.; Fieberg, J.R. Circular-linear copulae for animal movement data. Methods Ecol. Evol. 2022, 13, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Signer, J.; Fieberg, J.; Avgar, T. Animal movement tools (amt): R package for managing tracking data and conducting habitat selection analyses. Ecol. Evol. 2019, 9, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Killeen, J.; Thurfjell, H.; Ciuti, S.; Paton, D.; Musiani, M.; Boyce, M.S. Habitat selection during ungulate dispersal and exploratory movement at broad and fine scale with implications for conservation management. Mov. Ecol. 2014, 2, 15. [Google Scholar] [CrossRef]
- Liu, X.; Xu, N.; Jiang, A. Tortuosity entropy: A measure of spatial complexity of behavioral changes in animal movement. J. Theor. Biol. 2015, 364, 197–205. [Google Scholar] [CrossRef]
- Terbish, K.; Munkhbaatar, K.; Munkhbaatar, M. A Guide to the Amphibians and Reptiles of Mongolia; Boldgiv, B., Ed.; Admon Print Publishers: Ulaanbaatar, Mongolia, 2013. [Google Scholar]
- Zhao, K.T. Phrynocephalus Kaup. In Fauna Sinica, Reptilia, Vol. 2 (Squamata: Lacertilia); Zhou, K.Y., Zhao, K.T., Zhao, E.M., Eds.; Science Press: Beijing, China, 1999; pp. 151–192. [Google Scholar]
- Buehler, M.; Zoljargal, P.; Purvee, E.; Munkhbayar, K.; Munkhbaatar, M.; Batsaikhan, N.; Ananjeva, N.; Orlov, N.; Papenfuss, T.; Roldán-Piña, D.; et al. The results of four recent joint expeditions to the Gobi Desert: Lacertids and agamids. Russ. J. Herpetol. 2021, 28, 15–32. [Google Scholar] [CrossRef]
- Munkhbayar, K.; Munkhbaatar, M. Herpetological diversity of Mongolia and its conservation issues. Explor. Into Biol. Resour. Mong. 2012, 12, 203–212. [Google Scholar]
- Rogovin, K.; Semenov, D.; Shenbrot, G. Lizards of the northern Mongolian deserts: Densities and community structure. Asiat. Herpetol. Res. 2001, 9, 113–121. [Google Scholar]
- Yadamsuren, O.; Murdoch, J.; Chuluunbat, S.; Purevee, E.; Munkhbayar, M.; Jargalsaikhan, A.; Purevjargal, Z.; Khorloo, M.; Khayankhyarvaa, T. Estimating occupancy and detectability of toad headed agamas at the periphery of their range in Mongolia. J. Herpetol. 2018, 52, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Aguilar-Gómez, D.; Brandt, D.; Square, T.; Li, J.; Liu, Z.; Wang, T.; Sudmant, P.; Miller, C.; Nielsen, R. Population genomics of variegated toad-headed lizard Phrynocephalus versicolor and its adaptation to the colorful sand of the Gobi Desert. Genome Biol. Evol. 2022, 14, evac076. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Li, J.; Wo, Y.; Shao, G.; Zhao, W.; Aguilar-Gómez, D.; Jin, Y. Effects of substrate color on intraspecific body color variation in the toad-headed lizard, Phrynocephalus versicolor. Ecol. Evol. 2019, 9, 10253–10262. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Liu, Z.; Wang, T.; Yang, M.; Li, J.; Sun, H.; Niu, C.; Zhao, W.; Jin, Y. Camouflage versus running performance as strategies against predation in a lizard inhabiting different habitats. Ecol. Evol. 2021, 11, 17409–17416. [Google Scholar] [CrossRef]
- Hu, Q.; Lin, Y.; Qiu, X.; Fu, J.; Qi, Y. High-elevation adaptation of motion visual display modifications in the toad-headed agamid lizards (Phrynocephalus). Asiat. Herpetol. Res. 2022, 13, 53–63. [Google Scholar] [CrossRef]
- Gorin, V.A.; Dunayev, E.A.; Vassiliev, B.D. Step by step examination of tail movement sequences reveals functional differentiation in signals of spotted toad-headed agamas Phrynocephalus guttatus (Gmelin, 1789) (Reptilia: Agamidae). Russ. J. Herpetol. 2023, 30, 56–64. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, X.; Fu, J.; Peters, R.; Qi, Y. Testing the factors on the evolution of movement-based visual signal complexity in an Asian agamid lizard. Behav. Ecol. Sociobiol. 2023, 77, 135. [Google Scholar] [CrossRef]
- Qi, Y.; Noble, D.W.A.; Wu, Y.; Whiting, M.J. Sex- and performance-based escape behaviour in an Asian agamid lizard, Phrynocephalus vlangalii. Behav. Ecol. Sociobiol. 2014, 68, 2035–2042. [Google Scholar] [CrossRef]
- Qiu, X.; Fu, J.; Qi, Y. Tail waving speed affects territorial response in the toad-headed agama Phrynocephalus vlangalii. Asiat. Herpetol. Res. 2018, 9, 182–187. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, Q.; Peters, R.; Yue, B.; Fu, J.; Qi, Y. Unraveling the content of tail displays in an Asian agamid lizard. Behav. Ecol. Sociobiol. 2021, 75, 117. [Google Scholar] [CrossRef]
- Zhu, X.; Ya, Z.; Qi, Y. Tail display intensity is restricted by food availability in an Asian agamid lizard (Phrynocephalus vlangalii). Asian Herpetol. Res. 2020, 11, 240–248. [Google Scholar] [CrossRef]
- Eifler, D.A.; Eifler, M.A. Movement and habitat use by adult and juvenile toad-headed agama lizards (Phrynocephalus versicolor Strauch, 1876) in the eastern Gobi Desert, Mongolia. Herpetol. Notes 2019, 12, 717–719. [Google Scholar]
- Murdoch, J.; Suuri, B.; Reading, R. Estimates of toad headed agama density in three steppe habitats of Mongolia. Explor. Into Biol. Resour. Mong. 2010, 11, 383–389. [Google Scholar]
- Peters, R.A.; Ramos, J.A.; Hernandez, J.; Wu, Y.; Qi, Y. Social context affects tail displays by Phrynocephalus vlangalii lizards from China. Sci. Rep. 2016, 6, 31573. [Google Scholar] [CrossRef]
- Cooper, W.E. Multiple roles of tail display by the curly-tailed lizard Leiocephalus carinatus: Pursuit deterrent and deflective roles of a social signal. Ethology 2001, 107, 1137–1149. [Google Scholar] [CrossRef]
- Font, E.; Carazo, P.; Lanuza, G.P.; Kramer, M. Predator-elicited foot shakes in wall lizards (Podarcis muralis): Evidence for a pursuit-deterrent function. J. Comp. Psychol. 2012, 126, 87–96. [Google Scholar] [CrossRef]
- Eifler, M.A.; Marchand, R.; Eifler, D.A.; Malela, K. Habitat use and activity patterns in the nocturnal gecko, Chondrodactylus turneri. Herpetologica 2017, 73, 43–47. [Google Scholar] [CrossRef]
- Tryban, M.E.; Utsumi, K.L.; Olson, C.N.; Yang, J.L.; Reynolds, H.; Eifler, M.A.; Eifler, D.A. Sex-based variation in behavior for the little striped whiptail (Aspidoscelis inornatus). Southwest. Nat. 2024, 68, 112–120. [Google Scholar] [CrossRef]
- Fisher, K.E.; Bradbury, S.P. Influence of habitat quality and resource density on breeding-season female monarch butterfly Danaus plexippus movement and space use in north-central USA agroecosystem landscapes. J. Appl. Ecol. 2022, 59, 431–443. [Google Scholar] [CrossRef]
- Hart, D. Foraging and resource patchiness–field experiments with a grazing stream insect. Oikos 1981, 37, 46–52. [Google Scholar] [CrossRef]
- Ord, T.J. Costs of territoriality: A review of hypotheses, meta-analysis, and field study. Oecologia 2021, 197, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Partecke, J.; Haeseler, A.; Wikelski, M. Territory establishment in lekking marine iguanas, Amblyrhynchus cristatus: Support for the hotshot mechanism. Behav. Ecol. Sociobiol. 2002, 51, 579–587. [Google Scholar] [CrossRef]
- Panov, E.N.; Tsellarius, A.Y.; Nepomnyashchikh, V.A. Motor coordinations in the behavior of the toad-headed agama, Phrynocephalus mystaceaus (Reptilia, Agamidae): Signal functions and endogenous rhythms. Entomol. Rev. 2004, 84 (Suppl. 2), S185–S194. [Google Scholar]
- Qi, Y.; Noble, D.W.A.; Fu, J.; Whiting, M.J. Spatial and social organization in a burrow-dwelling lizard (Phrynocephalus vlangalii) from China. PLoS ONE 2012, 7, e41130. [Google Scholar] [CrossRef]
- Anderson, R.A.; Karasov, W.H. Energetics of the lizard Cnemidophorus tigris and life history consequences of food-acquisition mode. Ecol. Monogr. 1988, 58, 79–110. [Google Scholar] [CrossRef]
- Cox, R.M.; Calsbeek, R. Severe costs of reproduction persist in Anolis lizards despite the evolution of a single-egg clutch. Evolution 2010, 64, 1321–1330. [Google Scholar] [CrossRef]
- Miles, D.B.; Sinervo, B.; Frankino, W.A. Reproductive burden, locomotor performance, and the cost of reproduction in free ranging lizards. Evolution 2000, 54, 1386–1395. [Google Scholar] [CrossRef]
- Bauer, A.M. Gekkonid lizards as prey of invertebrates and predators of vertebrates. Herpetol. Rev. 1990, 21, 83–87. [Google Scholar]
- Childers, J.L.; Eifler, D.A. Meroles cuneirostris (wedge-snouted sand lizard). Cannibalism. Herpetol. Rev. 2013, 44, 675–676. [Google Scholar]
- Liu, E.F.; Buchanan, C.A.; Eifler, M.A.; Eifler, D.A. Meroles anchietae (Anchieta’s shovel-snouted lizard). Cannibalism. Herpetol. Rev. 2019, 50, 580. [Google Scholar]
- Chiaverano, L.M.; Wright, M.J.; Holland, B.S. Movement behavior is habitat dependent in invasive Jackson’s chameleons in Hawaii. J. Herpetol. 2014, 48, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Turchin, P. Quantitative Analysis of Movement: Measuring and Modeling Population Redistribution in Animals and Plants; Sinauer Associates, Inc., Publishers: Sunderland, MA, USA, 1998. [Google Scholar]
- Yacelga, M.; Cayot, L.J.; Jaramillo, A. Dispersal of neonatal Galápagos marine iguanas Amblyrhynchus cristatus from their nesting zone: Natural history and conservation implications. Herpetol. Conserv. Biol. 2012, 7, 470–480. [Google Scholar]
- Ehlman, S.M.; Scherer, U.; Bierbach, D.; Stark, L.; Beese, M.; Wolf, M. Developmental arcs of plasticity in whole movement repertoires of a clonal fish. iScience 2025, 28, 113189. [Google Scholar] [CrossRef]
- Colaço, J.R.; Araujo, H.A.; da Luz, M.G.E.; Viswanathan, G.M.; Bartumeus, F.; Raposo, E.P. Effect of the search space dimensionality for finding close and faraway targets in random searches. Phys. Rev. E 2022, 106, 034124. [Google Scholar] [CrossRef]
- Kembro, J.M.; Lihoreau, M.; Garriga, J.; Raposo, E.P.; Bartumeus, F. Bumblebees learn foraging routes through exploitation-exploration cycles. J. R. Soc. Interface 2019, 16, 20190103. [Google Scholar] [CrossRef]
- Moore, T.Y.; Cooper, K.L.; Biewener, A.A.; Vasudevan, R. Unpredictability of escape trajectory explains predator evasion ability and microhabitat preference of desert rodents. Nat. Commun. 2017, 8, 440. [Google Scholar] [CrossRef]
- Pagés, J.F.; Bartumeus, F.; Romero, J. Alcoverro, T. The scent of fear makes sea urchins go ballistic. Mov. Ecol. 2021, 9, 50. [Google Scholar] [CrossRef]
- Riotte-Lambert, L.; Benhamou, S.; Chamaillé-Jammes, S. From randomness to traplining: A framework for the study of routine movement behavior. Behav. Ecol. 2017, 28, 280–287. [Google Scholar] [CrossRef]
- Vasconcelos, R.; Santos, X.; Carretero, M.A. High temperatures constrain microhabitat selection and activity patterns of the insular Cape Verde wall gecko. J. Arid. Environ. 2012, 81, 18–25. [Google Scholar] [CrossRef]
- Vidal, M.; Ortiz, J.C.; Labra, A. Sexual and age differences in ecological variables of the lizard Microlophus atacamensis (Tropiduridae) from northern Chile. Rev. Chil. Hist. Nat. 2002, 75, 283–292. [Google Scholar] [CrossRef]
- Cook, E.G.; Murphy, T.G.; Johnson, M.A. Colorful displays signal male quality in a tropical anole lizard. Naturwissenschaften 2013, 100, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Labra, A.; Carazo, P.; Desfilis, E.; Font, E. Agonistic interactions in a Liolaemus lizard: Structure of head bob displays. Herpetologica 2007, 63, 11–18. [Google Scholar] [CrossRef]
- Braun, C.A.; Baird, T.A. Collared lizard juveniles use caudal displays while stalking prey. J. Herpetol. 2018, 52, 113–115. [Google Scholar] [CrossRef]
- Cooper, W.E. Pursuit deterrence in lizards. Saudi J. Biol. Sci. 2000, 7, 15–29. [Google Scholar]
- Eifler, D.A.; Eifler, M.A. Characteristics and use of the tail in signaling by the zebra-tailed lizard (Callisaurus draconoides). Southwest. Nat. 2010, 55, 104–109. [Google Scholar] [CrossRef]
- Bian, X.; Zhao, W.; Qi, Y.; Peters, R. Tail tales: How ecological context mediates signal effectiveness in a lizard. Integr. Zool. 2025; early view. [Google Scholar] [CrossRef]
- Ord, T.J.; Peters, R.A.; Evans, C.S.; Taylor, A.J. Digital video playback and visual communication in lizards. Anim. Behav. 2002, 63, 879–890. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Qi, Y. Links between variation in movement-based visual signals and social communication complexity in an Asian agamid lizard Phrynocephalus vlangalii. Animals 2025, 15, 38. [Google Scholar] [CrossRef]
- Ma, M.; Luo, S.; Tang, X.; Chen, Q. Age structure and growth pattern of a high-altitude lizard population based on age determination by skeletochronology. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 491–500. [Google Scholar] [CrossRef]
- Zhang, K.; Tong, H.; Wo, Y.; Liu, N.; Jin, Y. Sex ratio and sexual size dimorphism in a mad-headed lizard, Phrynocephalus guinanensis. Asian Herpetol. Res. 2018, 9, 35–42. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, N. The proximate causes of sexual size dimorphism in Phrynocephalus przewalskii. PLoS ONE 2014, 9, e85963. [Google Scholar] [CrossRef]
- Herbert-Read, J.E.; Ward, A.J.W.; Sumpter, D.J.T.; Mann, R.P. Escape path complexity and its context dependency in Pacific blue-eyes (Pseudomugil signifer). J. Exp. Biol. 2017, 220, 2076–2081. [Google Scholar] [CrossRef]
- Fleming, C.H.; Subaşi, Y.; Calabrese, J.M. Maximum-entropy description of animal movement. Phys. Rev. E 2015, 91, 032107. [Google Scholar] [CrossRef]
- Postlethwaite, C.M.; Brown, P.; Dennis, T.E. A new multi-scale measure for analysing animal movement data. J. Theor. Biol. 2013, 317, 175–185. [Google Scholar] [CrossRef]



| Behavior | Definition | |
|---|---|---|
| Behavior | Dig | The anterior feet and legs displace substrate material, creating a depression. |
| Eat | Consumption of food involves opening and closing the mouth before swallowing. | |
| Move | A change in location of the body involving a displacement of ≥1 body length. | |
| Tail display | Tail raised by curling upwards, then straightening, exposing the ventral surface. Often repeated in a rapid series. | |
| Movement Path | Step length | Straight-line distance between consecutive 30 s markers. |
| Path length | Sum of all step lengths for an observation period. | |
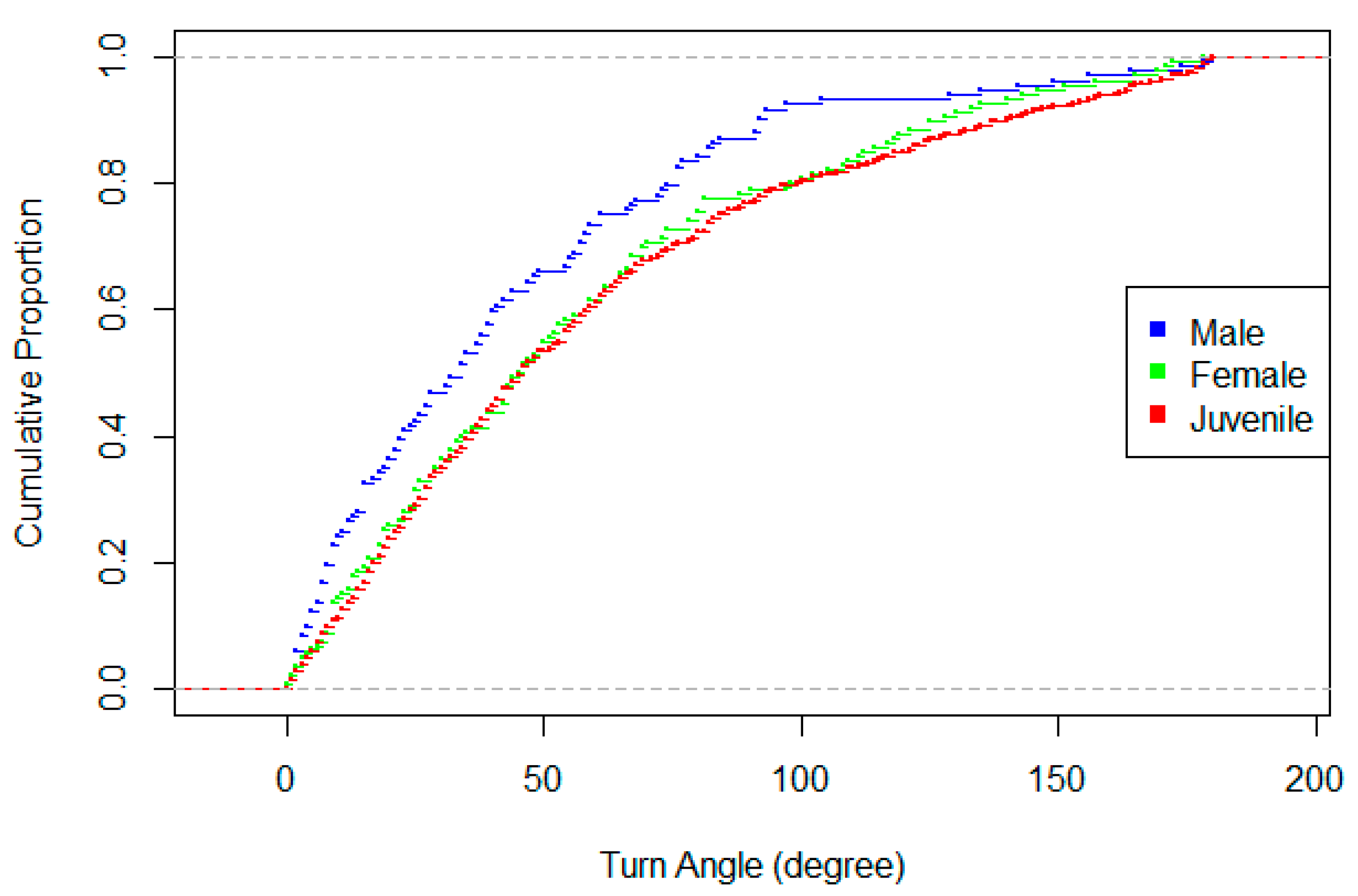
| Turn angle | Change in direction between consecutive step lengths. Possible values = 0–180°, with 0° = an unchanged orientation by the focal animal from the previous step. | |
| Search area | Size of the area (minimum convex polygon) occupied during the observation. |
| Value (units) | Males (n = 21; 18) | Females (n = 31; 29) | Juv (n = 61; 53) | Statistic |
|---|---|---|---|---|
| SVL (mm) | 52.3 ± 0.6 a | 50.2 ± 0.5 b | 30.6 ± 0.4 c | 671 (<0.001) |
| Mass (g) | 5.9 ± 0.2 a | 5.2 ± 0.1 b | 1.2 ± 0.1 c | 625 (<0.001) |
| Tail length/SVL | 1.28 (1.04–1.37) a | 1.19 (1.07–1.38) b | 1.29 (1.15–1.43) a | 27.23 (<0.0001) |
| Dig (n) | 0 (0–4) a | 0 (0–3) a | 1 (0–27) b | 24.41 (<0.001) |
| Eat (n) | 0 (0–9) a | 0 (0–10) a | 3 (0–20) b | 25.6 (<0.001) |
| Tail display (n) | 0 (0–14) a | 0 (0–8) a | 9 (0–42) b | 30.65 (<0.001) |
| Move (n) | 19.5 (0–49) a | 7 (0–36) a | 35 (10–110) b | 42.58 (<0.001) |
| No moves (n intervals) | 17.5 (0–30) a | 25 (12–30) a | 17 (2–28) b | 21.82 (<0.001) |
| Path length (cm) | 382 (0–4658) a,b | 174 (0–1813) a | 646 (34–4569) b | 13.73 (0.001) |
| Search area (m2) | 0.9 (0–171.8) a,b | 0.33 (0–36.89) a | 3.32 (0–130.34) b | 13.45 (0.001) |
| Entropy | 0.77 (0–1.99) a | 0.42 (0–1.74) a | 1.41 (0.35–2.36) b | 25.96 (<0.001) |
| Value (units) | F-M | F-Juv | M-Juv | |
|---|---|---|---|---|
| Tail/SVL | 3.52 (0.0004) | 5.09 (<0.0001) | 0.44 (0.66) | |
| Behavior | Dig (n) | 0.80 (0.42) | 4.62 (<0.001) | 3.07 (0.002) |
| Eat (n) | 0.56 (0.57) | 4.64 (<0.001) | 3.31 (<0.001) | |
| Tail display (n) | 0.74 (0.46) | 5.14 (<0.001) | 3.48 (<0.001) | |
| Movement | Move (n) | 1.26 (0.21) | 6.18 (<0.001) | 3.85 (<0.001) |
| No moves (n intervals) | 1.58 (0.11) | 4.60 (<0.001) | 2.16 (0.03) | |
| Path length (cm) | 1.26 (0.21) | 3.65 (<0.001) | 1.70 (0.09) | |
| Search area (m2) | 1.26 (0.21) | 3.62 (<0.001) | 1.67 (0.09) | |
| Entropy | 0.27 (0.79) | 4.55 (<0.001) | 3.56 (<0.001) |
| Body Size | Behavior | Movement | ||||||
|---|---|---|---|---|---|---|---|---|
| SVL | Eat | Dig | Tail Display | Moves | 0 MovInt | Path Length | Search Area | |
| Eat | −0.50 (0.04) | |||||||
| Dig | −0.46 (0.05) | 0.31 (0.21) | ||||||
| Tail display | −0.27 (0.34) | −0.08 (0.79) | 0.52 (0.05) | |||||
| Moves | −0.36 (0.14) | 0.54 (0.02) | 0.66 (0.003) | 0.40 (0.14) | ||||
| 0 MovInt | 0.42 (0.08) | −0.45 (0.06) | −0.37 (0.13) | −0.25 (0.36) | −0.59 (0.01) | |||
| Path length | −0.23 (0.36) | 0.50 (0.03) | 0.56 (0.02) | 0.52 (0.05) | 0.85 (<0.001) | −0.56 (0.02) | ||
| Search area | −0.19 (0.45) | 0.47 (0.05) | 0.45 (0.06) | 0.31 (0.26) | 0.67 (0.003) | −0.47 (0.05) | 0.95 (<0.001) | |
| Entropy | −0.42 (0.08) | 0.7 (0.001) | 0.47 (0.05) | 0.36 (0.19) | 0.91 (<0.001) | −0.58 (0.01) | 0.69 (0.002) | 0.48 (0.04) |
| Body Size | Behavior | Movement | ||||||
|---|---|---|---|---|---|---|---|---|
| SVL | Eat | Dig | Tail Display | Moves | 0 MovInt | Path Length | Search Area | |
| Eat | −0.14 (0.47) | |||||||
| Dig | −0.26 (0.19) | 0.19 (0.34) | ||||||
| Tail display | −0.51 (0.009) | 0.21 (0.31) | −0.07 (0.74) | |||||
| Moves | −0.31 (0.10) | 0.58 (0.001) | 0.12 (0.53) | 0.47 (0.02) | ||||
| 0 MovInt | 0.29 (0.12) | −0.59 (0.001) | −0.06 (0.78) | −0.38 (0.06) | −0.95 (<0.001) | |||
| Path length | −0.31 (0.10) | 0.30 (0.12) | 0.14 (0.48) | 0.14 (0.51) | 0.63 (<0.001) | −0.55 (0.002) | ||
| Search area | −0.14 (0.49) | 0.017 (0.93) | 0.01 (0.96) | −0.01 (0.96) | 0.23 (0.23) | −0.13 (0.51) | 0.87 (<0.001) | |
| Entropy | −0.13 (0.51) | 0.53 (0.003) | 0.10 (0.62) | 0.22 (0.29) | 0.86 (<0.001) | −0.91 (<0.001) | 0.56 (0.002) | 0.15 (0.44) |
| Body Size | Behavior | Movement | ||||||
|---|---|---|---|---|---|---|---|---|
| SVL | Eats | Digs | Tail Display | Moves | 0 MovInt | Path Length | Search Area | |
| Eat | 0.04 (0.78) | |||||||
| Dig | 0.02 (0.87) | −0.19 (0.18) | ||||||
| Tail display | −0.20 (0.19) | 0.17 (0.25) | 0.25 (0.09) | |||||
| Moves | −0.12 (0.39) | 0.40 (0.003) | 0.02 (0.90) | 0.50 (<0.001) | ||||
| 0 MovInt | 0.07 (0.61) | −0.31 (0.02) | −0.04 (0.78) | −0.44 (0.002) | −0.85 (<0.001) | |||
| Path length | 0.01 (0.92) | 0.06 (0.67) | −0.08 (0.60) | 0.26 (0.08) | 0.73 (<0.001) | −0.67 (<0.001) | ||
| Search area | 0.06 (0.69) | −0.03 (0.86) | −0.12 (0.41) | 0.11 (0.48) | 0.59 (<0.001) | −0.46 (0.001) | 0.93 (<0.001) | |
| Entropy | −0.04 (0.76) | 0.14 (0.31) | 0.13 (0.35) | 0.47 (0.001) | 0.64 (<0.001) | −0.79 (<0.001) | 0.42 (0.002) | 0.26 (0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utsumi, K.; Pham, A.; Erdenetsetseg, B.; Eifler, M.; Eifler, D. Demographic Differences in Behavior, Movement, and Habitat Use in the Toad-Headed Agama (Phrynocephalus versicolor) of the Gobi Desert (Dornogovi, Mongolia). Diversity 2025, 17, 659. https://doi.org/10.3390/d17090659
Utsumi K, Pham A, Erdenetsetseg B, Eifler M, Eifler D. Demographic Differences in Behavior, Movement, and Habitat Use in the Toad-Headed Agama (Phrynocephalus versicolor) of the Gobi Desert (Dornogovi, Mongolia). Diversity. 2025; 17(9):659. https://doi.org/10.3390/d17090659
Chicago/Turabian StyleUtsumi, Kaera, Alicia Pham, Batdelger Erdenetsetseg, Maria Eifler, and Douglas Eifler. 2025. "Demographic Differences in Behavior, Movement, and Habitat Use in the Toad-Headed Agama (Phrynocephalus versicolor) of the Gobi Desert (Dornogovi, Mongolia)" Diversity 17, no. 9: 659. https://doi.org/10.3390/d17090659
APA StyleUtsumi, K., Pham, A., Erdenetsetseg, B., Eifler, M., & Eifler, D. (2025). Demographic Differences in Behavior, Movement, and Habitat Use in the Toad-Headed Agama (Phrynocephalus versicolor) of the Gobi Desert (Dornogovi, Mongolia). Diversity, 17(9), 659. https://doi.org/10.3390/d17090659






