Abstract
The chestnut gall wasp (Dryocosmus kuriphilus Yasumatsu) is an invasive pest that attacks species of the genus Castanea, inducing gall formation on buds and leaves, which can significantly reduce tree growth, fruiting, and overall chestnut production. Native to China, D. kuriphilus has become a serious threat to chestnut orchards worldwide. Torymus sinensis Kamijo, a parasitoid also originating from China, is highly specific to D. kuriphilus and is currently considered the most effective biological control agent against this pest. This study aimed to evaluate the establishment of T. sinensis as well as its effectiveness in controlling D. kuriphilus at release sites between 2020 and 2023. Releases of T. sinensis were conducted in the municipality of Bragança with a sex ratio of 120 females to 70 males. The parasitoids were randomly released across three chestnut trees with infestation levels ranging from moderate (26–50% of the canopy affected by galls) to very severe (>80% of the canopy affected). At each release site, 250 galls were collected annually, and 10% of these galls were dissected to calculate parasitism rates by T. sinensis. Results revealed a positive correlation between the monitoring year and the parasitism rate. Following the releases, parasitism rates increased gradually, reaching values between 15% and 40%. T. sinensis successfully established itself in chestnut orchards and parasitized D. kuriphilus, despite normal population fluctuations being observed across years and orchards.
1. Introduction
The parasitoid Torymus sinensis Kamijo, 1982 (Hymenoptera: Torymidae), is recognized as a specific biological control agent for the chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu. This pest induces the formation of galls on buds, leaves, and petioles, leading to tree weakening and significantly impacting tree production. Losses can reach up to 80% by the fourth year of infestation [1]. Native to China, D. kuriphilus has become a serious threat to chestnut orchards worldwide.
T. sinensis is a univoltine species, with females laying an average of 70 eggs during their lifespan of approximately 30 days. Fertilized eggs develop into diploid females, while unfertilized eggs produce haploid males [2,3]. In early spring, T. sinensis females oviposit within newly formed D. kuriphilus galls, typically laying one egg per host larva. The parasitoid larva hatches shortly thereafter, consuming its host by late spring before entering dormancy. Pupation occurs in late winter, and adults emerge in early spring of the following year, coinciding with chestnut tree sprouting and the formation of new D. kuriphilus galls [2,3].
The first introduction of T. sinensis occurred in Japan in 1975, and it was subsequently released in all regions where D. kuriphilus had become established. These include the USA (late 1970s), Italy (2002), France (2011), Croatia (2014), Hungary (2014), Bosnia and Herzegovina (2016), Spain (2015), Portugal (2015), and Slovenia (2015) [2,4,5,6,7,8]. In Europe, T. sinensis was first introduced in Italy in 2002, marking the beginning of its use as a biological control agent on the continent [6].
The release of T. sinensis has proven to be the most effective strategy for controlling D. kuriphilus. Its success depends on a carefully planned release process, which requires synchronization of the pest and parasitoid life cycles. This process also relies on comprehensive knowledge of D. kuriphilus biology, chestnut tree phenology, and the influence of climatic conditions on both pest development and crop growth [9].
Monitoring efforts in countries where T. sinensis has been released reveals considerable variability in parasitism rates. For example, in Campania, Italy, parasitism rates ranged from 0.5% to 164% between 2009 and 2016 [10]. Similarly, monitoring conducted in Bosnia and Herzegovina in 2017 showed rates between 44.83% and 74% [4].
In Portugal, the national action plan for the control of D. kuriphilus, coordinated by the Direção Geral de Agricultura e Veterinária (DGAV), outlines guidelines for biological control, including monitoring programs for both D. kuriphilus and T. sinensis, as well as protocols for the release of T. sinensis [1].
The objective of this study was to evaluate the parasitism rates of T. sinensis during the release year and their evolution over the subsequent four years.
2. Materials and Methods
2.1. Torymus sinensis Releases
Over four years (2020–2023), a total of 851 T. sinensis releases were conducted in chestnut orchards across 36 civil parishes in the municipality of Bragança. The release sites were spaced at least 500 m apart and selected based on the absence of prior releases and the presence of moderate (26–50% of the canopy of three trees with galls) to severe (>80% of the canopy of three trees with galls) infestations. Each release involved a sex ratio of 120 females to 70 males, distributed in 11 tubes (3 cm in diameter and 12 cm in length).
The release process was conducted on days without rain or wind to minimize interference with parasitoid activity. Parasitoids were randomly distributed among three heavily infested chestnut trees. The tubes were opened, and insects were gently stimulated to exit onto the leaves, ensuring their dispersal into the orchard.
2.2. Monitoring the Effectiveness of T. sinensis
The effectiveness of T. sinensis releases was monitored during winter (January) each year from 2021 to 2024. Monitoring was conducted at 25% of the release sites for each year (45 sites in 2020, 56 in 2021, 52 in 2022, and 50 in 2023), as well as at previously monitored sites from earlier years (5 sites in 2020, 15 in 2021, 29 in 2022, and 37 in 2023).
At each monitored site, a total of 250 galls were collected from five to six randomly selected chestnut trees [1]. The samples were taken from branches located at various heights (1.5–3 m above the ground) to ensure representative sampling.
The collected galls were transported to the laboratory, where they were cleaned with a brush and stored at room temperature in emergence boxes (Figure 1) until adult insects emerged. These boxes were constructed from cardboard (32 cm × 27 cm × 10 cm) and equipped with two collection tubes (3 cm in diameter and 12 cm in length) for emerging insects. After all insects had emerged, the boxes were opened, and adults were collected and identified using a binocular microscope and identification keys.
Figure 1.
(A)—Emergence boxes (B)—Dryocosmus kuriphilus Yasumatsu galls harvesting.
Subsequently, 10% of the collected galls were dissected to count the number of larval chambers. The parasitism rate was calculated using Equation (1).
2.3. Statistical Analysis
The success of T. sinensis releases was evaluated by calculating the median number of T. sinensis individuals captured, as well as the average parasitism rates and their standard errors for each civil parish. Linear regression analyses and ANOVA tests were performed to assess the relationship between the number of T. sinensis released and the number subsequently captured, as well as the relationship between the year of release and parasitism rates. The homogeneity of variance test (Levene’s test) was also carried out on the data analyzed.
To carry out the ANOVA test to assess the relationship between the number of parasitoids released and those collected, it was necessary to group the data into three categories: Low (100–599), Medium (600–999), and High (1000–4000).
All statistical analyses and calculations were conducted using Microsoft Excel (Microsoft Office LTSC Professional Plus 2021), PAST, version 0.45 (http://www.uio.no/õhammer/past accessed on 15 March 2025), and JASP, version 0.19.3 (https://jasp-stats.org/download/ accessed on 15 March 2025).
3. Results
The number of releases conducted in each chestnut orchard and the average parasitism rates of T. sinensis are presented in Table 1.

Table 1.
Released, collected, and parasitism rate of T. sinensis in 36 chestnut orchards located in the municipality of Bragança, Portugal.
Over the four years of the study, a total of 851 releases were performed, introducing 161,690 T. sinensis individuals into the municipality of Bragança. This included 102,120 mated females (Table 1). During the release year (n0, corresponding to 2020, 2021, 2022, and 2023), 38,570 T. sinensis individuals were introduced across all sites, with 32,725 individuals recovered during monitoring in the same release year. In total, 50,750 galls were collected across all monitoring events.
The recovery rate of parasitoids was positively associated with the number of releases conducted. Linear regression analysis (Figure 2) revealed a positive correlation (R2 = 0.8068, F = 136.152, p = 0.080254) between the number of T. sinensis released and the number recovered. The R2 value indicated that 80.68% of the variation in the dependent variable (Y), the number of T. sinensis recovered, was explained by the regression model, suggesting a relatively good fit to the data and a strong explanatory power.
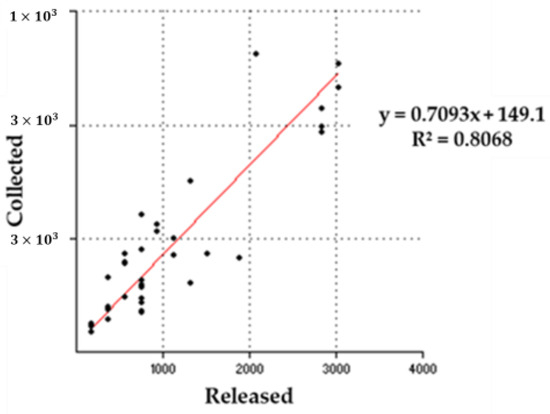
Figure 2.
Relationship between the number of Torymus sinensis Kamijo released and collected. The total number of adults released per site and adults that emerged from the galls are shown in the regression line (red line): y = 0.7093x + 149.1.
However, the p-value associated with the F-test was 0.080254 (p > 0.05), indicating that the model was not statistically significant. This suggests that while there is a clear trend in the data, the results may have been influenced by other factors not captured by the regression model or the variability in the data itself.
The one-way ANOVA test revealed a significant difference between the groups (F(2,33) = 19.11, p < 0.001). The collected averages were higher in the High group (M = 1602.1; SD = 743.2) than in Medium (M = 701.9; SD = 316.3) and Low (M = 444.5; SD = 246.5) groups. Tukey’s post hoc tests confirmed that High differed from Medium and Low (p < 0.05) and that Medium differed from Low (p < 0.05). (more information in Supplementary Materials).
Average parasitism rates in the release year (n0, corresponding to 2020, 2021, 2022, and 2023) varied significantly across civil parishes, ranging from 7.37% in Carragosa to 33% in Grijó de Parada (Table 1).
Monitoring of releases (Figure 3) revealed parasitism rates that fluctuated between 0.5% (Carregosa) and 31.5% (Coelhoso) in 2020 (A), 0.7% (Parâmio) and 49.4% (Quintela de Lampaças) in 2021 (B), 3.1% (Sendas) and 41.1% (Pinela) in 2022 (C), and 0.1% (Mós) and 59.8% (Rebordainhos) in 2023 (D). These results indicate variability in parasitism rates over the years and among locations, reflecting potential differences in environmental conditions, release success, or local population dynamics of D. kuriphilus and T. sinensis.
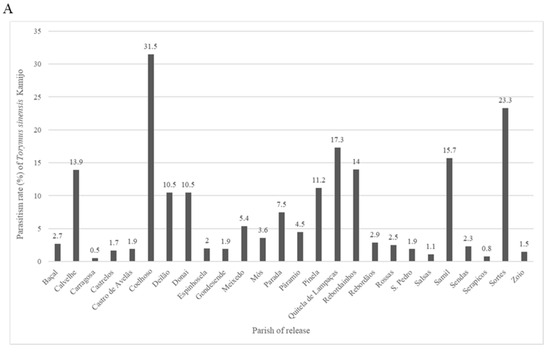
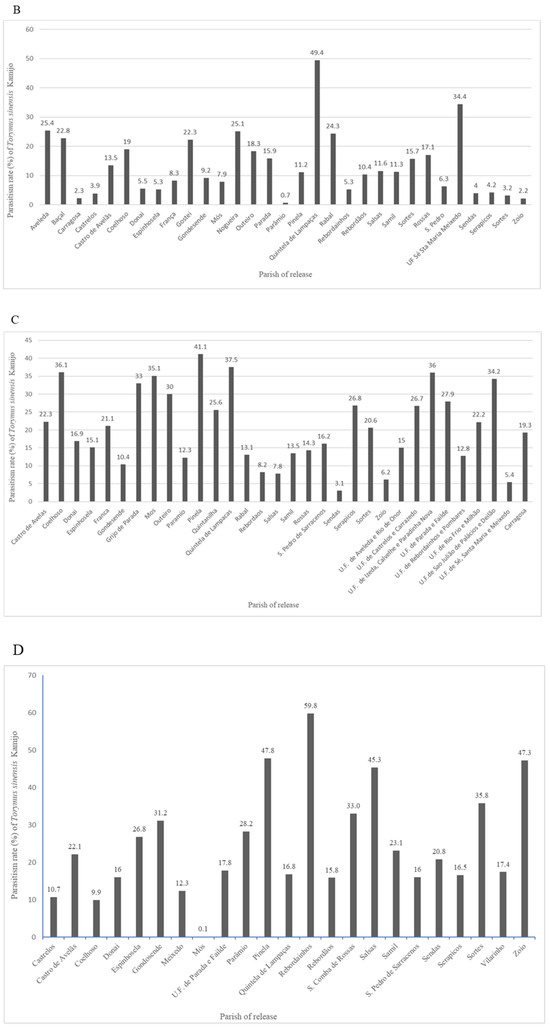
Figure 3.
Torymus sinensis Kamijo parasitism rates in the release year (n0) ((A)—2020, (B)—2021, (C)—2022, (D)—2023), in the municipality of Bragança, Portugal.
For chestnut orchards monitored continuously over four years, parasitism rates exhibited year-to-year variations, generally showing an upward trend. However, declines were observed in Calvelhe and Pinela. Notably, the sampling in Coelhoso revealed a decrease in parasitism rates from 2021 to 2022, and by 2023, no D. kuriphilus galls were found in this orchard (Figure 4).
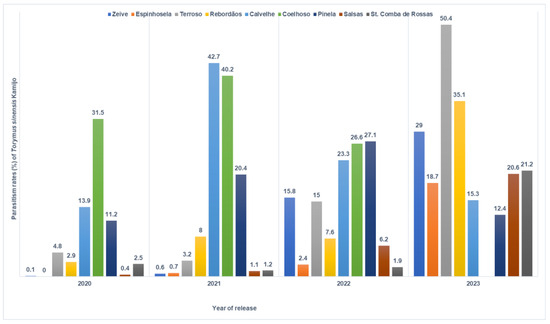
Figure 4.
Parasitism rates (%) of Torymus sinensis Kamijo in the chestnut orchards monitored over 4 years (2020, 2021, 2022, and 2023), in the municipality of Bragança.
A linear regression analysis (Figure 5) between the year of monitoring and the parasitism rate revealed a positive correlation (R2 = 0.1881; F = 7.647; p = 0.009). The R2 value indicates that 18.81% of the variation in the parasitism rate is explained by the model. Although this value suggests limited explanatory power, it implies that other factors not included in the model may be influencing the parasitism rate.
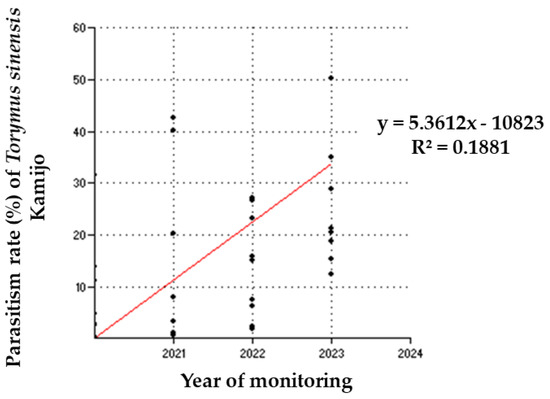
Figure 5.
Relationship between the year of monitoring and the parasitism rate of Torymus sinensis Kamijo. The parasitism rates per site and the year of monitoring are shown in the regression line (red line): y = 5.3612x − 10823.
The F-value is moderate, and the associated p-value of 0.009 is below the 0.05 significance threshold. This confirms that the relationship between the year and the parasitism rate is statistically significant at a 5% significance level, albeit with a limited ability to fully explain the observed variability.
There was no significant effect of year on the parasitism rate (ANOVA: F(3.30) = 2.45, p = 0.082). The average parasitism rates were 8.4% in 2020 and 25.3% in 2023, but the variation between years was not statistically significant. The assumption of equal variances was met (Levene p = 0.210) (more information in Supplementary Materials).
4. Discussion
The observed increase in T. sinensis populations over the years aligns with expectations, as this parasitoid requires time to establish and expand its population. Previous studies indicate that T. sinensis typically needs 5–7 years after the initial release or settlement to achieve high parasitism rates [11]. For example, in Japan, the first country to introduce T. sinensis, dispersal rates were estimated at less than 1 km/year during the initial years [11].
Fluctuations in natural parasitism rates over the years, along with the limited effectiveness of native parasitoids, support the importance of introducing T. sinensis as a biocontrol agent. Its efficacy has proven to be significant, delivering high levels of pest control within a few years post-introduction [11].
In the municipality of Bragança, during the release year (n0) between 2020 and 2023, the average number of T. sinensis individuals collected ranged from 45 to 664 per civil parish. This compares favorably with Campania, Italy, where T. sinensis recovery ranged from 9 to 212 individuals [10].
Table 1 highlights considerable variability in parasitism rates across civil parishes. Similar variability has been observed in other countries and is often attributed to factors such as climatic conditions, agricultural practices, and the natural acclimatization of T. sinensis [10].
The relationship between the number of T. sinensis released and recovered (Figure 2) showed that higher recovery rates were generally associated with larger release efforts. Comparable trends were observed in Campania, Italy, where larger releases correlated with greater parasitoid recovery [10].
Parasitism rates of T. sinensis in some civil parishes of Bragança tended to increase over the four years (2020–2023), except in Calvelhe and Pinela, where rates declined. Notably, in Coelhoso, no Dryocosmus kuriphilus galls were found for monitoring in 2023, suggesting a significant reduction in pest presence. The effectiveness of T. sinensis has been consistently documented in other countries, particularly in Italy, where studies report positive trends in parasitism rates and long-distance movements of T. sinensis with parasitism rates ranged from 0.5% to 164% between 2009 and 2016 [4,5,10].
The linear regression analysis of parasitism rates and monitoring years (Figure 5) revealed a positive relationship between these variables. Following releases, parasitism rates increased gradually, ranging between 15% and 40%.
This study demonstrates that T. sinensis successfully colonized chestnut orchards in Bragança and parasitized D. kuriphilus. Despite normal population fluctuations observed across years and locations, the biocontrol agent showed its potential for managing the chestnut gall wasp effectively. Continued monitoring and release programs are recommended to further enhance T. sinensis establishment and long-term pest suppression.
5. Conclusions
The results of this study show that the releases of the parasitoid T. sinensis in chestnut orchards in the municipality of Bragança were successful in colonizing and controlling D. kuriphilus. The parasitism rate observed increased progressively over the four years of monitoring, reaching average values of between 15% and 40% in the different study sites, proving the ability of T. sinensis to establish and disperse in the conditions of the Trás-os-Montes region. Despite the spatial and temporal variability, influenced by differences in local climate, initial infestation status, and the density of releases, the general trend of increasing parasitism rates confirms the potential of this parasitoid as a biocontrol agent for this pest.
In addition, the positive correlation (R2 = 0.81) between the number of specimens released and the number recovered reinforces the importance of a continuous and well-sized release effort to accelerate the consolidation of the T. sinensis population. The lack of statistical significance in some years or specific locations highlights the need for adjustments to the release strategy, namely in the choice of locations, the ideal time, and the density of releases, in order to optimize the effectiveness of biological control.
In practical terms, it is recommended to maintain this release program associated with a regular monitoring plan, allied to sustainable agronomic practices (maintaining plant diversity and reducing chemical pesticides), in order to boost the long-term suppression of D. kuriphilus in the Trás-os-Montes chestnut orchards. Future research should explore the interaction of T. sinensis with native parasitoids, as well as the impact of extreme climatic variables on the effectiveness of the biocontrol, in order to guarantee robust and resilient control of this invasive pest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17050358/s1.
Author Contributions
A.B. and A.L.S. contributed to the conception and design of the study. Material preparation and data collection were carried out by A.L.S., R.M., J.M.-S., S.F. and V.G.; data analysis was carried out by A.L.S. and S.A.P.S. The first draft of the manuscript was written by A.L.S., A.B., S.A.P.S. and P.A.C., who revised and corrected the manuscript until it was finalized. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project: Bio4Med—Implementação de estratégias inovadoras para incremento da sustentabilidade em culturas perenes mediterrânicas (PRR-C05-i03-I-000083).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Direção Geral de Alimentação e Veterinária (DGAV). Plano de Ação Nacional Para Controlo Do Inseto Dryocosmus Kuriphilus; DGAV: Lisboa, Portugal, 2017; pp. 1–46. [Google Scholar]
- Cardoso, J.; Almeida, M.T.; Bento, A. Biological control of Dryocosmus kuriphilus Yasumatsu with the parasitoid Torymus sinensis Kamijo. Millenium J. Educ. Technol. Health 2021, 2, 91–99. [Google Scholar] [CrossRef]
- Amorim, A.; Rodrigues, R.; Nunes, L.J.R.; Freitas, M.; Moura, L. Dryocosmus Kuriphilus Yasumatsu (Hymenoptera: Cynipidae) in Minho (Northern Portugal): Bioecology, Native Parasitoid Communities and Biological Control with Torymus Sinensis Kamijo (Hymenoptera: Torymidae). Agronomy 2022, 12, 2184. [Google Scholar] [CrossRef]
- Matošević, D.; Mujezinović, O.; Dautbašić, M. First Record of Biocontrol Agent Torymus sinensis (Hymenoptera; Torymidae) in Bosnia and Herzegovina. South-East Eur. For. 2017, 8, 147–149. [Google Scholar] [CrossRef][Green Version]
- Matosevic, D.; Lacković, N.; Melika, G.; Kos, K.; Franić, I.; Kriston, E.; Boszo, M.; Seljak, G.; Rot, M. Biological Control of Invasive Dryocosmus kuriphilus with Introduced Parasitoid Torymus sinensis in Croatia, Slovenia and Hungary. Period Biol 2016, 117, 471–477. [Google Scholar] [CrossRef]
- Viciriuc, I.-M.; Mitroiu, M.-D.; Askew, R.R.; Ris, N.; Fusu, L.; Borowiec, N. Torymus Sinensis and Its Close Relatives in Europe: A Multilocus Phylogeny, Detailed Morphological Analysis, and Identification Key. Arthropod Syst. Phylogeny 2023, 81, 705–730. [Google Scholar] [CrossRef]
- Paparella, F.; Ferracini, C.; Portaluri, A.; Manzo, A.; Alma, A. Biological Control of the Chestnut Gall Wasp with T. sinensis: A Mathematical Model. Ecol. Model. 2016, 338, 17–36. [Google Scholar] [CrossRef]
- Gil-Tapetado, D.; López-Estrada, E.K.; Jiménez Ruiz, Y.; Cabrero-Sañudo, F.J.; Gómez, J.F.; Durán Montes, P.; Rey del Castillo, C.; Rodríguez-Rojo, M.P.; Polidori, C.; Nieves-Aldrey, J.L. Torymus sinensis against the Invasive Chestnut Gall Wasp: Evaluating the Physiological Host Range and Hybridization Risks of a Classical Biological Control Agent. Biol. Control 2023, 180, 105187. [Google Scholar] [CrossRef]
- Graziosi, I.; Rieske, L.K. Response of Torymus sinensis, a Parasitoid of the Gallforming Dryocosmus kuriphilus, to Olfactory and Visual Cues. Biol. Control 2013, 67, 137–142. [Google Scholar] [CrossRef]
- Cascone, P.; Carpenito, S.; Iodice, L.; Raimo, S.; Guerrieri, E. Introduction and Acclimation of Torymus sinensis in the South of Italy. Entomol. Gen. 2018, 37, 93–101. [Google Scholar] [CrossRef]
- Nieves-Aldrey, J.L.; Gil-Tapetado, D.; Gavira, O.; Boyero, J.R.; Polidori, C.; Lombardero, M.J.; Blanco, D.; Rey del Castillo, C.; Rodriguez Rojo, P.; Vela, J.M.; et al. Torymus sinensis Kamijo, a Biocontrol Agent against the Invasive Chestnut Gall Wasp Dryocosmus kuriphilus Yasumatsu in Spain: Its Natural Dispersal from France and the First Data on Establishment after Experimental Releases. For. Syst. 2019, 28, e001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).