Abstract
Parthenium hysterophorus L. (Asteraceae), commonly known as famine weed in South Africa, is one of the most invasive weeds worldwide, accountable for losses to biodiversity, agriculture, the economy, and the health of livestock and human beings. The spread of this weed is not easy to manage or reverse. With globalization, trade, and human movement, it will continue to spread, allowing its range to expand into new areas. This weed has serious adverse effects on rangeland and agricultural crop production. Because of its unique characteristics, its invasiveness and competitive success rates are attributed to its high reproductive ability, fast growth rate, tolerance to environmental stresses, climate change adaptability, allelopathic potential, and unpalatability to animals. Parthenium hysterophorus dominates diverse habitats by spreading quickly as a contaminant of grain and other crop products and through farm machinery. It is widely distributed and has become problematic in various countries, including South Africa. It has successfully invaded several provinces in the country since its introduction in 1880. Since plant invasions are not universal, this study aimed to review research that has been conducted on the aggressive weed P. hysterophorus to understand the ecological characteristics that enable its successful establishment, the economic costs associated with its detrimental impacts, and to briefly assess knowledge gaps to improve its management strategies.
1. Introduction
Invasive alien plants (IAPs) are recognized as one of the major threats to biodiversity, agriculture, health, and socio-economic livelihoods around the world [1,2,3]. The introduction of IAPs into a new habitat may be deliberate or accidental [1] and, subsequently, some have become naturalized, while others have become invasive [4]. In addition, climate change is thought to exacerbate the situation caused by weeds and invasive alien plant species globally in the future [5,6]. Thus, the success of IAPs is generally attributed to differences in ecological traits compared to native species [7].
In South Africa, rangeland covers over 70% of the land surface and supports domestic livestock and wildlife, but is usually invaded by hundreds of alien plant species [8]. It is estimated that over 383 invasive plant species must be controlled to prevent further damage [9], of which about 44 (11.5%) species of herbaceous plants are declared weeds or invaders [10]. Among the 44 species, Parthenium hysterophorus L., belonging to the family Asteraceae and commonly known as famine weed, has been identified as one of the most invasive noxious weeds. Famine weed causes ecosystem degradation with significant negative impacts on ecological functions, natural biodiversity, agricultural productivity, and human and animal health [11,12].
The nature and severity of the impacts of Parthenium hysterophorus on biodiversity loss and native species extinction are of global concern for the conservation of many natural and managed areas [13]. It is a weed plant native to Central America, South America, and Mexico [14], and has been introduced both accidentally and intentionally in many parts of the world, including many countries in Africa [15,16,17]. It is spreading at an alarming rate and has become a severe invader in the southern and eastern parts of the continent [12,18]. Reports from different countries suggest that Parthenium is still spreading, and African countries are at a high risk of invasion [15].
Parthenium hysterophorus was introduced accidentally into South Africa through imported pasture, grain seeds, and livestock feed [18,19]. It was first recorded in KwaZulu-Natal in 1880, but has only became prevalent from the 1980s onwards [16,18,20]. Currently, the weed is widely distributed and has become problematic. It is now an emerging and established weed in the country, and the core infestations are in KwaZulu-Natal and Mpumalanga, as well as the North-West and Limpopo Provinces [21]. Moreover, dense and extensive infestations occur along roadsides, watercourses, natural and man-made ecosystems, grassland habitats, woodlands, wildlife parks, crop fields, and yards [15,22].
Successful invasive plants have advantageous ecological traits (high reproductive capacities, high dispersal ability, range size, life history, geographical distribution, climatic tolerances, habitat types, environmental indicator values, edaphic preferences, and phenotypic plasticity) that support their invasiveness [23]. Not every invasive species shares all these characteristics. However, ecological traits best describe the alien’s success over native plants. Numerous ecological characteristics support the invasiveness and competitive advantage of this weed. In addition, allelochemicals released from this weed inhibit germination and the growth of co-occurring plants [24,25].
Due to its aggressive coverage and threat, P. hysterophorus has severe consequences for the agricultural economy, human health, and biodiversity. This has contributed to its selection as a species of concern in the country [16]. Previously listed as a Category 1 weed under the Conservation of Agricultural Resources Act (Act 43 of 1983), P. hysterophorus is now classified as a Category 1b species according to the National Environmental Management Biodiversity Act (NEMBA) regulations [17]. As this weed proliferates across the country, the present review highlights the critical ecological traits that promote its invasiveness and assesses the socio-economic impacts of this weed to understand to what extent it is detrimental and/or beneficial. The possible uses of this weed can help with effective management. However, there is a paucity of information on its benefits in South Africa, since efforts have not been made yet by the scientific community, the local people, and other stakeholders to investigate its potential benefits. Such information is essential for determining the need for either upgrading or downgrading the invasion status of the species as per regulations, particularly Section 70 of the South African Regulations of the National Environmental Management: Biodiversity Act (NEMBA; Act 10 of 2004). In this context, this study aimed to review research that has been conducted on the aggressive weed P. hysterophorus to understand the ecological characteristics that enable its successful establishment, the economic costs associated with its detrimental impacts, and to briefly assess knowledge gaps that need to be addressed to improve management strategies.
2. Taxonomy and Description
Parthenium hysterophorus L., commonly known as famine weed in South Africa, is an aggressive, invasive alien weed belonging to the Asteraceae family. Various common English names include congress grass, Demoina weed, ragweed Parthenium, Parthenium weed, carrot grass, false ragweed, bitterweed, Santa Maria, and feverfew. This family comprises over 1900 genera within the order Asterales and more than 32,000 known species of flowering plants distributed worldwide [26]. The genus Parthenium, to which P. hysterophorus belongs, comprises 16 species [27].
Parthenium hysterophorus is an annual herbaceous weed growing up to 1.5 m high at maturity (Figure 1a) with an erect, greenish, longitudinally hairy branched stem (Figure 1b) and a deep tap root system [15,28]. The stems are usually octangular and woody as the plants develop to maturity. The alternately arranged leaves are deeply lobed, simple with petioles, variable in shape and size, elliptic to obovate, about 4–5 cm long, and glossy dark green and hairless, with fine and numerous lateral veins and a margin with very fine, gland-tipped teeth [29]. The lower leaves are relatively large and deeply divided (bi-pinnatifid or bi-pinnatisect), whereas the upper leaves decrease in size and are less divided. The attractive flowers are whitish or pinkish and ephemeral. Numerous small flower heads are arranged in clusters at the tips of the branches. The fruit of P. hysterophorus, which is light brown in color in its young days and later turns dark brown when mature, takes on an oblate and ellipsoid shape [30]. The tiny seeds have two slender, spoon-shaped achenes, usually produced in each flower head (capitulum), and are dispersed with minimal effort [31].

Figure 1.
Illustration of Parthenium hysterophorus L. (a) Growing Parthenium weed; (b) whitish flowers [32].
3. Ecological Traits of Parthenium hysterophorus Invasiveness
The characteristics that increase the probability of Parthenium hysterophorus to invade new habitats successfully are related to its high seed production and dispersion, capability of spreading fast, high phenotypic plasticity, adaptability to new areas, tolerance to biotic and abiotic stresses, and allelopathic chemical properties that suppress crops and pasture plants [33,34].
3.1. Reproductive Potential
Parthenium hysterophorus is an invasive, noxious weed considered as one of the worst weeds in the world. The success of P. hysterophorus as an invasive weed lies in its ability to reproduce large amounts of seeds, which have a prolonged seed viability of up to five years [35]. A single mature plant can produce 25,000 seeds in its entire lifespan [36], leading to a large and persistent seed bank in the soil [37]. Parthenium hysterophorus has the potential for rapid germination and a fast growth rate. It grows vigorously during the warm spring and summer months compared with the winter months [37,38]. The large production of small, lightweight seeds with a wide dispersal range enables their fast spread and colonization [39]. The successful invasion of this weed also depends on its dispersion strategies. Parthenium hysterophorus seeds are dispersed by wind, water, animals, vehicles, attachment to packaging materials, tools, and clothing, which enables them to invade new areas quickly and extensively [35,40]. Moreover, most of the long-distance dispersal is through vehicles, farm machinery, and flooding [15].
According to Leishman et al. [41], the size and number of seeds produced by invasive plants affect the species’ ability to colonize new habitats and form a stable population. Parthenium hysterophorus seeds can germinate and establish new individuals in a variety of environmental conditions and different habitats [18,33,42,43]. It aggressively colonizes disturbed habitats; roadsides; pastures; agricultural areas; urban areas; industrial areas; playgrounds; and roadsides in semi-arid, subtropical, tropical, and warmer temperate regions [44].
Reproduction ability is one of the most crucial biological traits for the effectiveness of Parthenium as a weed [45]. The viability of the seeds and their dispersal are also major concerns for the successful invasion of this weed. Bajwa et al. [35] reported that the tiny seeds of this weed usually remain viable in the soil longer and form more permanent seed banks. Jeyalakshmi and Valluvaparidasan [46] emphasize that Parthenium seeds do not undergo a dormancy period, and they can germinate at any time when moisture is available. The spread of seeds and their ability to remain viable in the soil for a long period pose one of the most complex problems for control [47]. Bhowmik et al. [48] stated that the viability of Parthenium seeds under suitable environmental conditions is more than 85%. This weed can form a large seed bank at dense infestations, estimated at 200,000 seeds per m2, which usually remain viable in the soil longer [49,50]. Where conditions are favorable, the production of many seeds allows P. hysterophorus to infest crops heavily. Even though the reproductive process of P. hysterophorus conducted in other countries revealed that this weed species has wide ecological tolerance to germinate and grow in various environmental conditions successfully [33], it seems to be poorly documented in South Africa and, thus, needs to be investigated.
3.2. Distribution and Habitat Suitability
Parthenium hysterophorus has been reported in various countries [30,40], and many reports suggest its widespread distribution worldwide. Within the last few decades, this weed has expanded its geographic range and is threatening to invade much larger areas of the world [18,51]. The distribution and density of Parthenium are still increasing in many parts of the world, even in areas where it has been present for many years [35]. Research literature studies on ecological niche models suggest that this weed can perform better under climate change scenarios and expand into uninvaded regions in the future [11]. In Africa, its current and potential geographical distribution has been reported in Egypt [52] and several countries in eastern and southern Africa [14,17,18]. In South Africa, P. hysterophorus is particularly distributed in the KwaZulu-Natal, Mpumalanga, and North-West Limpopo Provinces [18,37,53]. It can also be found in Gauteng Province. The occurrence data for P. hysterophorus in South Africa, employed in this study, were sourced from the Global Biodiversity Information Facility (GBIF) [54]. It is, however, classified as an alien invader in Category 1b [55].
As this species has invaded the rangelands of these provinces, it has disrupted the natural balance of plant communities and, thus, has resulted in a reduction in plant diversity and the abundance of native plant species (Figure 2) [56]. Kriticos et al. [51] indicated the risk of a substantial further spread of P. hysterophorus under a climate change scenario. As Parthenium grows vigorously in semi-arid, subtropical, tropical, and warmer temperate regions [14,17,33,44], parts of South Africa experience high mean annual temperatures of approximately 28 °C. Hence, this weed is projected to spread further in other provinces because of the favorable climatic conditions of the country. It is highly adaptable to a wide range of environmental conditions, from wet to warm areas, and to different soil types, mostly sandy loam and sandy clay loam [57]. Its tolerance to various pH values intensifies its exponential growth and spread, especially to newly invaded and disturbed landscapes [58]. Its growth is best suited under high light intensities [59] and elevated nitrogen levels [60]. The high phenotypic plasticity allows this weed species to settle in novel surroundings via suitable biomass allocation [61,62]. It is a very common ruderal species, particularly prolific in disturbed habitats [21]. Because of its invasiveness, in many countries where it grows, including South Africa, it can establish luxuriantly in wastelands, roadsides, along railways, pastures, fallow land, seasonal floodplains, grasslands, riparian zones, woodlands, lawns, gardens, and agricultural fields [21,30,63].

Figure 2.
A dense thicket of famine weed engulfs farmland in KwaZulu-Natal Province, South Africa [56].
Like many other invasive species, Parthenium hysterophorus can establish and extensively expand its range under climate change [40,64] due to its tolerance to a wide range of environments, including high temperatures, extreme soil moisture, and increasing carbon dioxide (CO2) concentrations [64]. Despite potential Parthenium infestation risks in South Africa, only a few studies have documented Parthenium distribution [18]. Hence, there is a need to accurately predict the potential distribution of Parthenium in South Africa, as many reports from different countries suggest that this weed is still spreading and may become prominent in different terrestrial ecosystems of the world [35,65]. The local adaptability of invasive species like Parthenium to a wide range of environmental conditions and, thus, to more diverse habitats is an essential factor in its successful invasion. According to Cowie et al. [66], P. hysterophorus is a highly detrimental invasive species in South Africa, particularly in the savanna regions (Figure 3).
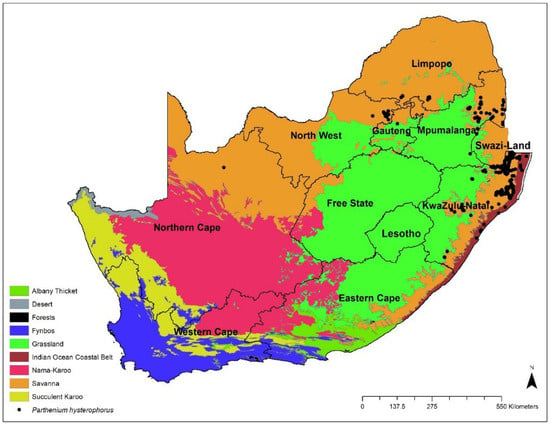
Figure 3.
Current distribution of Parthenium hysterophorus L in South Africa based on GBIF and SAPIA records (GBIF: Global Biodiversity Information Facility; SAPIA: Southern African Plant Invaders Atlas).
3.3. Allelopathy
Parthenium has been termed the worst invasive alien plant species, and it is considered a cropping, grazing, and environmental weed [67]. It can outcompete annual crops, causing tremendous yield loss and suppressing the growth of surrounding native species. [68]. Famine weed changes the community structure in invaded ecosystems, and the decline in species heterogeneity could possibly be due to its strong allelopathic effect on neighboring species. The release of allelopathic substances into the surrounding environment results in the formation of dense monospecies stands of the weed [68,69].
Allelopathy is a phenomenon observed in many invasive plant species that involves the release of secondary compounds by a plant that affects the growth of surrounding plants in a stimulatory and inhibitory manner. Hassan et al. [70] reported that releasing allelochemicals in low amounts stimulates growth, while greater amounts result in the inhibition of surrounding plants. Allelopathy is one of the fundamental mechanisms responsible for the success of invasive alien plant species. The success of aggressive plant species such as Parthenium concerning invasion and establishment in new areas is thought to involve their ability to suppress and replace other species within the existing plant community [71]. Phenolics and sesquiterpene lactones are two major classes of allelopathic compounds produced by Parthenium, and these chemicals are known to have a significant adverse effect on the germination, seedling emergence, and growth of various plant species [67]. The allelopathic properties of different parts of this weed have been well demonstrated in the germination and seedling growth of surrounding plant species.
Several laboratory experiments conducted under laboratory conditions have tested the allelopathic effect of the weed by applying the aqueous extract directly to test plants under natural conditions. For instance, Imad et al. [72] revealed that the aqueous leaf extract of P. hysterophorus significantly reduced germination and suppressed the growth parameters of the tested species. Similarly, Bashar et al. [25] showed that the allelopathic potential of leaf, stem, and flower extracts of P. hysterophorus on the germination and seedling growth of selected plants and weeds decreased drastically. Furthermore, Netsere and Mendesil [73] reported that the aqueous extracts of the shoot, leaf, flower, and root of Parthenium hysterophorus inhibited the germination of crops (Glycine max L. and Phaseolus vulgaris L.). Under natural conditions, Putri [67] examined the allelopathic effect of Parthenium leaf litter and reported that wheat growth reduction (Triticum aestivum L.) increased as the amount of Parthenium leaf litter increased. Similarly, Khosla and Sobti [74] reported that Parthenium caused a yield decrease of 40% in crops and up to a 90% reduction in forage production in grasslands in India.
It was reported in eastern Ethiopia that sorghum grain yield was reduced by between 40 and 97% in different seasons due to this weed [75]. In South Africa, if uncontrolled, Parthenium has the potential to reduce crop yields by as much as 97% [37] due to poisonous phenolics and lactones produced by the leaves [18,24].
4. Socio-Economic Impacts of Parthenium Invasion
Invasive alien species are a major threat to biodiversity and human well-being, resulting in ecosystem degradation and causing economic costs globally. Parthenium hysterophorus is of great concern among the invasive alien plant species identified in South Africa. It is considered one of the worst weeds responsible for causing several ecological and agricultural problems, such as the loss of crop productivity, fodder scarcity, health problems for human beings and livestock, and biodiversity depletion [66,67,76]. Parthenium is progressively spreading in the country and covering vast areas in KwaZulu-Natal and Mpumalanga, as well as the North-West, Limpopo, and Gauteng Provinces (Figure 3), resulting in a negative impact on crop production, native biodiversity, livestock feed supply, and the health of the local people. The range of socio-economic problems caused by this weed impacts the lives of local communities and national economic development. Studies in various countries have shown that the impact of the Parthenium invasion on animal and human health and the economic loss in agriculture are becoming severe [16].
With the increase in global trade, the transfer of invasive alien plants with both beneficial and deleterious impacts will continue [77]. Therefore, the geographic extent of IAPs such as P. hysterophorus will increase, leading to enormous costs to ecosystems, the economy, and society [78]. The harmful effects of P. hysterophorus are well documented in several studies (Table 1); however, the weed does have several benefits (Table 1) [79]. In South Africa, Parthenium is classified as an alien invader in Category 1b [55], and the scientific community, local people, and other stakeholders do not view the weed as having benefits. Plants in this category are prohibited and must be controlled. They serve no economic purpose and possess characteristics that are harmful to humans, animals, and the environment [17].

Table 1.
Harmful and useful effects of Parthenium hysterophorus.
5. Effect of Parthenium hysterophorus on Biodiversity
Parthenium hysterophorus has been ranked as one of the most problematic weeds in the world, since it poses serious challenges to biodiversity [99]. The ability of this weed to occupy available niches has reduced the availability of habitats for the local flora, which affects the biodiversity of the invaded areas [100]. Parthenium hysterophorus can easily outcompete native grasses, causing fodder shortages for livestock. Several studies have shown that high seed production in Parthenium enhances its spread and it replaces native plant species by creating homogenous stands [13,50]. The release of allelochemicals by this weed, which inhibits the germination and growth of various crops and native plant species, is essential for its invasion and survival in a wide range of native and non-native habitats, resulting in ecosystem change [101]. Several studies have documented the effects of P. hysterophorus on the diversity and abundance of native plant species [13,50,102].
In Ethiopia, the impact of P. hysterophorus on grazing land communities showed that the diversity and evenness of native plant species reduced as the density of P. hysterophorus increased, implying that the heterogeneity of the community was significantly affected [102]. A negative relationship between the density of P. hysterophorus and the species richness and abundance of native species was also recorded [13]. Several researchers have observed the dominance of Parthenium in grassland ecosystems.
In another study conducted in Kenya, the effects of P. hysterophorus on herbaceous plant species diversity revealed that P. hysterophorus significantly reduced the species diversity and richness of the native plant species. These adverse effects of P. hysterophorus on plant species diversity and abundance are likely to lower the availability of pasture species, as reported by Nguyen et al. [11]. Furthermore, the weed has changed the habitat in native Australian grasslands [92]. Even though studies conducted in different parts of the world revealed the aggressiveness of P. hysterophorus on native plant species, data on the impacts of P. hysterophorus on the species richness, evenness, diversity, and composition of native plants in invaded communities are limited in South Africa. It is recommended that future studies focus on the restoration of P. hysterophorus in an invaded savanna using perennial grass seed.
6. Effect of Parthenium hysterophorus on Crops and Livestock Production
Parthenium hysterophorus severely decreases the recruitment of native species, causes a yield decline, and affects livestock productivity by reducing the amount of forage. Moreover, it increases agricultural production costs and causes environmental damage [85,103]. It is also known to be poisonous and unpalatable to livestock due to its odor, taste, and the presence of trichomes, which affects milk production and the quality of meat [15,43,79]. Ojija et al. [104] reported that this weed negatively affects foraging behavior and flower visitation in bees. Its invasion in Ethiopia has not only devastated crop production, but has also resulted in grazing shortages, since the weed is unpalatable to livestock [105]. In Pakistan, the farming communities of different cropping regions in rural Punjab reported significant effects of Parthenium on their cropland and livestock production [43]. In Australia, it has become a major weed on grazing lands in central Queensland and New South Wales [106]. Invasions by P. hysterophorus severely degrade and remove valuable grazing land, reducing the carrying capacity of pastures and limiting livestock and game production [102].
Crop production is the main source of food and means of income for most of the people living in the KwaZulu-Natal, Mpumalanga, North-West, and Limpopo Provinces, where these weed species have naturalized. The infestation of P. hysterophorus in these provinces may have a detrimental effect on crop and livestock production. Even though some scholars have reported its presence in South Africa’s rangelands [16,37,53], few studies have documented the detrimental invasion of Parthenium on agricultural lands and livestock production to date.
7. Impact of Parthenium hysterophorus on Human and Livestock Health
The spread of P. hysterophorus may have deleterious impacts on human and livestock health in different communities. Bhowmik et al. [48] reported that the weed is toxic to humans, as well as cattle. When humans encounter this weed, it may cause dermatitis, nausea, eczema, bronchitis, black spots and blisters around the eyes, and skin redness [79]. Inhalation of the pollen can cause allergenic rhinitis, which can develop into bronchitis and asthma if the pollen enters the respiratory tract during breathing. Evans [85] reported that 10% to 20% of the human population have developed severe allergic reactions after being exposed to the weed for a period of 1 to 10 years, based on research conducted in Australia. Based on their study in Islamabad, Shabbir and Bajwa [107] suggested that Parthenium may cause pollen allergy and high fever. The health of animals feeding on this weed is also affected.
Some studies have reported that animals that ate this weed were affected by continuous diarrhea and weight loss [79]. A study conducted in India on the toxicity of the weed to cattle and buffalo showed that a significant amount (10–50%) of the weed in the diet can kill animals within 30 days [108]. Similarly, Tudor et al. [109] reported taints in meat from sheep grazing in Parthenium-invaded Australian grasslands. Furthermore, TNAU [110] reported that cattle walking or grazing on patches of this weed were affected by an inflamed udder and, subsequently, suffered from fever and rashes. According to Singh et al. [111] and Kaur et al. [112], Parthenium compounds are lethal to humans and animals.
8. Management Approaches to Control Parthenium
Some invasive alien plants (IAPs) can positively affect several areas, including agriculture, the ornamental horticulture industry, and wood production. However, their use can result in harmful effects, representing a conflict of interest in their management [113]. Due to the degree of their environmental, economic, social, and aesthetic impacts, harmful IAPs such as P. hysterophorus need to be controlled. Despite the aggressive effects on crops and livestock production, the control of P. hysterophorus is difficult. Parthenium hysterophorus produces many seeds that have prolonged seed viability [35]. The weed has a rapid growth rate and a preference for a wide range of ecological conditions for its establishment [15,101].
All these ecological traits have been highlighted as factors contributing to the invasion success of P. hysterophorus [101]. Additionally, global climate change is predicted to increase P. hysterophorus invasions in the future [11,43]. Hence, the eradication of this weed might be challenging and expensive. This highlights not only the severity of the weed, but also the importance of developing strategies for its control. However, to control P. hysterophorus, several methods, including physical, chemical, and biological management approaches with varying degrees of success, have been used in many parts of the world [15,114]. Among the chemical methods to control Parthenium, chlorimuron ethyl, glyphosate, atrazine, ametryn, bromoxynil, and metsulfuron are known to be very effective [15]. According to Kaur et al. [15], chemical management is the most widely used method to control the growth of Parthenium hysterophorus, and physical methods, such as manual hand plucking before flowering and seed setting, are the most effective. However, manual hand plucking does not seem to be recommended, since it might cause serious health hazards. There is evidence to suggest that high populations of Parthenium, even if removed from the field, could potentially have lasting allelopathic effects on crop germination and seedling establishment. Burning Parthenium is ineffective and not recommended because it can stimulate its regeneration, posing a great risk to soil, plant, and animal diversity. Biological control might be a useful approach for controlling this weed, using insects that feed on the weed.
Parthenium can be managed by its natural enemies like insects, fungi, nematodes snails, slugs, and competitive plants [115]. Currently, about nine insect species have been introduced to control Parthenium [116], of which Zygogramma bicolorata and Epiblema strenuana have shown a strong capacity to manage this weed [15]. In addition, Puccinia abrupta var. partheniicola and phytoplasma of faba bean phyllody are the two most important pathogens causing disease to P. hysterophorus [117]. Another important management approach that has shown potential in many countries to control Parthenium is by planting other competitive plant species such as Cassia sericea, C. tora, C. auriculata, Croton bonplandianum, Amaranthus spinosus, Tephrosia purpurea, Hyptis suaveolens, Sida spinosa, and Mirabilis jalapa [118]. Some plant species from South Africa, namely, Panicum maximum, Digitaria eriantha, Imperata cylindrica, Cenchrus pennisetiformis, and Sorghum halepense, have been recorded to effectively compete with Parthenium [24].
Even though the spread of Parthenium has been successfully managed in Australia and India using biological agents such as insects, pathogens, and competitive indigenous plant species [17], Kaur et al. [15] suggested that an integrated approach should be a better way to manage this noxious weed. Hence, the integrated method should be implemented effectively to manage this weed in South Africa.
9. Conclusions
This review showed the ecological characteristics that promote the success of Parthenium hysterophorus in various habitats and environmental conditions. The invasiveness and competitive advantage of this weed are supported by several traits, such as its ability to adapt to a wide range of climatic conditions, its numerous seed production, its high capacity for dispersion and growth, its large seed bank in the soil, its capability of spreading fast, its allelopathic effect, its short life cycle, its high phenotypic plasticity, and its tolerance to biotic and abiotic stresses. Hence, its proliferation is progressing at an alarming rate in South Africa and it has become a severe invader in many provinces. In the next few coming years, the invasion by P. hysterophorus is likely to aggressively expand in various vegetation types in South Africa, including grassland and savanna biomes. This study demonstrated that P. hysterophorus outcompetes native and crop plants and transmits diseases, resulting in social instability and economic hardship. Management approaches are important to prevent the further introduction and spread of this weed in new areas that are not yet infested in the country. Further studies on its socioeconomic impact are crucial and should be regularly assessed. It is also recommended that future studies focus on the phenotypic variants of P. hysterophorus and the impacts of its debris/residues on other plant and soil properties.
Author Contributions
M.A.P.T. contributed to the study conception and design, as well as the writing of the original draft and editing; P.W.M., K.K.N., M.C.M., L.J.R. and A.A.C. contributed to the writing of the original draft and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the Botany Department at North-West University for their contribution to preparing this review paper.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Rai, P.K. Environmental degradation by invasive alien plants in the Anthropocene: Challenges and prospects for sustainable restoration. Anthr. Sci. 2022, 1, 5–28. [Google Scholar] [CrossRef]
- Duncan, P.; Podest, E.; Esler, K.J.; Geerts, S.; Lyons, C. Mapping invasive herbaceous plant species with Sentinel-2 satellite imagery: Echium plantagineum in a Mediterranean shrubland as a case study. Geomatics 2023, 3, 328–344. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.; Tiwari, A.K.; Singh, R.; Patel, S.K.; Singh, G.S. Perceptions of impacts and management of invasive alien plants: A case study from Mirzapur, India. Front. For. Glob. Change 2023, 6, 1194076. [Google Scholar] [CrossRef]
- Wilson, J.R.U.; Ivey, P.; Manyama, P.; Nänni, I. A new national unit for invasive species detection, assessment and eradication planning. S. Afr. J. Sci. 2013, 109, 13. [Google Scholar] [CrossRef]
- Sheppard, C.S.; Burns, B.R.; Stanley, M.C. Future-proofing weed management for the effects of climate change: Is New Zealand underestimating the risk of increased plant invasions? N. Z. J. Ecol. 2016, 40, 398–405. [Google Scholar] [CrossRef]
- Adhikari, P.; Jeon, J.Y.; Kim, H.W.; Shin, M.-S.; Adhikari, P.; Seo, C. Potential impact of climate change on plant invasion in the Republic of Korea. J. Ecol. Environ. 2019, 43, 36. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Maurel, N. Characteristics of successful alien plants. Mol. Ecol. 2015, 24, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.G.; van Wilgen, B.W. The Impact of Invasive Alien Plants on Rangelands in South Africa. In Biological Invasions in South Africa. Invading Nature; Springer Series in Invasion Ecology; van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Springer: Cham, Switzerland, 2020; p. 14. [Google Scholar]
- Van Wilgen, B.W. The management of invasive alien plants in South Africa: Strategy, progress and challenges. Outlooks Pest Manag. 2018, 29, 13–17. [Google Scholar] [CrossRef]
- Henderson, L. Plant invaders of southern Africa. In Plant Protection Research Institute Handbook 5; Line Drawings, Maps and Descriptions of 161 Alien spp.; Agricultural Research Council: Pretoria, South Africa, 1995. [Google Scholar]
- Nguyen, T.; Bajwa, A.A.; Belgeri, A.; Navie, S.; O’Donnell, C.; Adkins, S. Impact of an invasive weed, Parthenium hysterophorus, on a pasture community in south-east Queensland, Australia. Environ. Sci. Pollut. Res. Int. 2017, 24, 27188–27200. [Google Scholar] [CrossRef]
- Tabe Ojong, M.P., Jr.; Alvarez, M.; Ihli, H.J.; Becker, M.; Heckelei, T. Action on invasive species: Control strategies of Parthenium hysterophorus L. on smallholder farms in Kenya. Environ. Manag. 2022, 69, 861–870. [Google Scholar] [CrossRef]
- Boja, M.; Girma, Z.; Dalle, G. Impacts of Parthenium hysterophorus L. on plant species diversity in Ginir District, Southeastern Ethiopia. Diversity 2022, 14, 675. [Google Scholar] [CrossRef]
- Shabbir, A.; Zalucki, M.P.; Dhileepan, K.; Khan, N.; Adkins, S.W. The current and potential distribution of Parthenium weed and Its biological control agent in Pakistan. Plants 2023, 12, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Aggarwal, N.K.; Kumar, V.; Dhiman, R. Effects and management of Parthenium hysterophorus: A weed of global significance. Int. Sch. Res. Not. 2014, 2014, 368647. [Google Scholar] [CrossRef] [PubMed]
- Terblanche, C.; Nänni, I.; Kaplan, H.; Strathie, L.W.; McConnachie, A.J.; Goodall, J.; Van Wilgen, B.W. An approach to the development of a national strategy for controlling invasive alien plant species: The case of Parthenium hysterophorus in South Africa. Bothalia 2016, 46, a2053. [Google Scholar] [CrossRef]
- Rubaba, O.; Chimbari, M.; Mukaratirwa, S. Scope of research on Parthenium hysterophorus in Africa. S. Afr. J. Plant Soil. 2017, 34, 323–332. [Google Scholar] [CrossRef]
- McConnachie, A.J.; Strathie, L.W.; Mersie, W.; Gebrehiwot, L.; Zewdie, K.; Abdurehim, A.; Abrha, B.; Araya, T.; Asaregew, F.; Assefa, F.; et al. Current and potential geographical distribution of the invasive plant Parthenium hysterophorus (Asteraceae) in eastern and southern Africa. Weed Res. 2011, 51, 71–84. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Richardson, D.M.; Wilson, J.R. Ornamental plants as invasive aliens: Problems and solutions in Kruger National Park, South Africa. Environ. Manag. 2008, 41, 32–51. [Google Scholar] [CrossRef]
- Strathie, L.W.; Cowie, B.W.; McConnachie, A.J.; Chidawanyika, F.; Musedeli, J.N.; Sambo, S.M.C.; Magoso, E.X.; Gareeb, M. A Decade of biological control of Parthenium hysterophorus L. (Asteraceae) in South Africa Reviewed: Introduction of insect agents and their status. Afr. Entomol. 2021, 29, 809–836. [Google Scholar] [CrossRef]
- Strathie, L.W.; McConnachie, A.J. History and Management—Southern Africa and Western Indian Ocean islands. In Parthenium Weed: Biology, Ecology and Management; Adkins, S., Shabbir, A., Dhileepan, K., Eds.; CABI International: Wallingford, UK, 2019; pp. 264–286. [Google Scholar]
- Duguma, G.; Fitamo, D.; Kebede, F. Socioeconomic and ecological consequences of Parthenium weed (Parthenium hysterophorus L.) in Boset Woreda, Ethiopia. Afr. J. Agric. Res. 2019, 14, 1921–1942. [Google Scholar]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef]
- Dukpa, R.; Tiwari, A.; Kapoor, D. Biological management of allelopathic plant Parthenium sp. Open Agric. 2020, 5, 252–261. [Google Scholar] [CrossRef]
- Bashar, H.M.K.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Uddin, M.K.; Asib, N.; Anwar, M.P.; Rahaman, F.; Haque, M.A.; Hossain, A. Evaluation of allelopathic effects of Parthenium hysterophorus L. methanolic extracts on some selected plants and weeds. PLoS ONE 2023, 6, e0280159. [Google Scholar] [CrossRef]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef]
- Flora of North America Editorial Committee. Flora of North America North of Mexico. St. Louis, Missouri and Cambridge, Massachusetts, USA: Missouri Botanical Garden and Harvard University Herbaria. 2018. Available online: http://www.efloras.org/flora_page.aspx?flora_id=1 (accessed on 2 December 2023).
- O’Donnell, C.; Adkins, S.W. Management of Parthenium weed through competitive displacement with beneficial plants. Weed Biol. Manag. 2005, 5, 77–79. [Google Scholar] [CrossRef]
- Costello, B.; Osunkoya, O.O.; Sandino, J.; Marinic, W.; Trotter, P.; Shi, B.; Gonzalez, F.; Dhileepan, K. Detection of Parthenium weed (Parthenium hysterophorus L.) and its growth stages using artificial intelligence. Agriculture 2022, 12, 1838. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). Parthenium hysterophorus L. Asteraceae—Parthenium weed. Data sheets on invasive alien plants. Bull. OEPP 2014, 44, 474–478. Available online: https://www.eppo.int/ (accessed on 15 January 2024). [CrossRef]
- Adkins, S.; Shabbir, A.; Dhileepan, K. Parthenium Weed—Biology, Ecology and Management; Adkins, S., Shabbir, A., Dhileepan, K., Eds.; CABI International: Boston, MA, USA, 2019. [Google Scholar] [CrossRef]
- NEMBA (National Environmental Management: Biodiversity Act). Act No. 10 of 2004, Alien and Invasive Species Lists, 2020; Government Gazette; Department of Environment, Forestry and Fisheries, SA: Pretoria, South Africa, 2020.
- Adkins, S.; Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ali, S.; Khan, M.; Hamayun, M.; Moon, Y.-S. Parthenium hysterophorus’s Endophytes: The second layer of defense against biotic and abiotic Stresses. Microorganisms 2022, 10, 2217. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef]
- Van der Laan, M.; Reinhardt, C.F.; Belz, R.G.; Truter, W.F.; Foxcroft, L.F.; Hurl, E.K. Interference potential of the perennial grasses Eragrostis curvula, Panicum maximum and Digitaria eriantha with Parthenium hysterophorus. Trop. Grassl. 2008, 42, 88–95. [Google Scholar]
- Cowie, B.W. Parthenium hysterophorus: Understanding the Invasion and Potential Controls. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2020. [Google Scholar]
- Pandey, D.K.; Palni, L.M.S.; Joshi, S.C. Growth, Reproduction, and Photosynthesis of Ragweed Parthenium (Parthenium hysterophorus). Weed Sci. 2003, 51, 191–201. [Google Scholar] [CrossRef]
- Horvitz, N.; Wang, R.; Wan, F.H.; Nathan, R. Pervasive human-mediated large-scale invasion: Analysis of spread patterns and their underlying mechanisms in 17 of China’s worst invasive plants. J. Ecol. 2017, 105, 85–94. [Google Scholar] [CrossRef]
- Mao, R.; Shabbir, A.; Adkins, S. Parthenium hysterophorus: A tale of global invasion over two centuries, spread and prevention measures. J. Environ. Manag. 2021, 279, 111751. [Google Scholar] [CrossRef] [PubMed]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The Evolutionary Ecology of Seed Size. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI International: Wallingford, UK, 2000; pp. 31–57. [Google Scholar]
- Kaur, A.; Batish, D.R.; Kaur, S.; Singh, H.P.; Kohli, R.K. Phenological behaviour of Parthenium hysterophorus in response to climatic variations according to the extended BBCH scale. Ann. Appl. Biol. 2017, 171, 316–326. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Adkins, S.W. Germination ecology of two Australian biotypes of ragweed parthenium (Parthenium hysterophorus) relates to their invasiveness. Weed Sci. 2018, 66, 62–70. [Google Scholar] [CrossRef]
- Kohli, R.K.; Batish, D.R.; Singh, H.; Dogra, K.S. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biol. Invasions 2006, 8, 1501–1510. [Google Scholar] [CrossRef]
- Mutua, B.M.; Chiuri, W.; Ngure, V.; Kimani, V. Distribution of parthenium weed (Parthenium hysterophorus) in Nakuru County, Kenya. J. Environ. Sci. Technol. 2022, 1, 48–57. [Google Scholar] [CrossRef]
- Jeyalakshmi, C.; Valluvaparidasan, V. Distribution of microorganisms in the Parthenium weed infested soil of Tamil Nadu. J. Biopestic. 2010, 3, 523. [Google Scholar]
- Monaco, J.T.; Weller, S.C.; Ashton, F.M. Weed Biology and Ecology, 4th ed.; Academic Publisher: New York, NY, USA, 2001. [Google Scholar]
- Bhowmik, P.; Sarkar, D.; Yaduraju, N. The status of Parthenium hysterophorus and its potential management. Ecoprint Int. J. Ecol. 2007, 14, 1–17. [Google Scholar] [CrossRef]
- Goodall, J.; Braack, M.; de Klerk, J.; Keen, C. Study on the early effects of several weed-control methods on Parthenium hysterophorus L. Afr. J. Range Forage Sci. 2010, 27, 95–99. [Google Scholar] [CrossRef]
- Royimani, L.; Mutanga, O.; Odindi, J.; Zolo, K.S.; Sibanda, M.; Dube, T. Distribution of Parthenium hysterophorus L. with variation in rainfall using multi-year SPOT data and random forest classification. Remote Sens. Appl. Soc. Environ. 2019, 13, 215–223. [Google Scholar]
- Kriticos, D.J.; Brunel, S.; Ota, N.; Fried, G.; Oude Lansink, A.G.J.M.; Panetta, F.D.; Prasad, T.V.; Shabbir, A.; Yaacoby, T. Downscaling pest risk analyses: Identifying current and future potentially suitable habitats for Parthenium hysterophorus with particular reference to Europe and North Africa. PLoS ONE 2015, 10, e0132807. [Google Scholar] [CrossRef] [PubMed]
- CABI International. Parthenium hysterophorus (Parthenium weed). In Invasive Species Compendium. 2014. Available online: http://www.cabi.org/isc/datasheet/45573 (accessed on 5 February 2024).
- Mbatha, S.K. Parthenium hysterophorus Distribution and Efficacy of Control in Hluhluwe-Imfolozi Park, Northern KwaZulu-Natal. Master’s Thesis, School of Life Sciences College of Agriculture, Engineering and Science University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2020; 82p. [Google Scholar]
- GBIF.org. GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0121039-200613084148143 (accessed on 1 January 2024).
- NEMBA. National Environmental Management: Biodiversity Act 2004 (Act No. 10 of 2004); Government Gazette 584; NEMBA: Pretoria, South Africa, 2014; No. 37320.
- Bega, S. South Africa (SA) Faces Famine Threat from Toxic Alien Weed. Independent Online (IOL) Cape Town, South Africa. 2017. Available online: https://www.iol.co.za/news/south-africa/sa-faces-famine-threat-from-toxic-alien-weed-7709810 (accessed on 1 January 2024).
- Rizwana, A.Q.; Syed, A.G.; Muhammad, A.G.; Kishwar, N.S. Effects of Parthenium hysterophorus L. on soil characteristics initially inhabited to Cannabis sativa L. Pak. J. Biol. Sci. 2006, 9, 2794–2797. [Google Scholar] [CrossRef]
- Ayele, S. The Impact of Parthenium (Parthenium hysterophorus L.) on the Range Ecosystem Dynamics of the Jijiga Rangeland, Ethiopia; Department of Animal Sciences, School of Graduate Studies, Haramaya University: Haramaya, Ethiopia, 2007; 134p. [Google Scholar]
- Navie, S.C.; Panetta, F.D.; McFadyen, R.E.; Adkins, S.W. Behaviour of buried and surface-sown seeds of Parthenium hysterophorus. Weed Res. 1998, 38, 335–341. [Google Scholar] [CrossRef]
- Singh, V. Effect of nitrogen addition on the architecture and biomass allocation of two invasive plant species (Ageratum conyzoides L. and Parthenium hysterophorus L). Proc. Natl. Acad. Sci. India B 2014, 81, 348–358. [Google Scholar]
- Kaur, A.; Kaur, S.; Singh, H.P.; Datta, A.; Chauhan, B.S.; Ullah, H.; Kohli, R.K.; Batish, D.R. Ecology, Biology, Environmental Impacts, and Management of an Agro-Environmental Weed Ageratum conyzoides. Plants 2023, 12, 2329. [Google Scholar] [CrossRef]
- Rathee, S.; Ahmad, M.; Sharma, P.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kaur, A.; Yadav, S.S.; Kohli, R.K. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 2021, 184, 104392. [Google Scholar] [CrossRef]
- Chetty, S.; Mutanga, O.; Lottering, R. Detecting and mapping invasive Parthenium hysterophorus L. along the northern coastal belt of KwaZulu-Natal, South Africa using image texture. Sci. Afr. 2021, 13, e00966. [Google Scholar] [CrossRef]
- Navie, S.; McFadyen, R.; Panetta, F.; Adkins, S. The effect of CO2 enrichment on the growth of a C3 weed (Parthenium hysterophorus L.) and its competitive interaction with a C4 grass (Cenchrus ciliaris L.). Plant Prot. Q. 2005, 20, 61–66. [Google Scholar]
- Dhileepan, K. Managing Parthenium Weed across Diverse Landscapes: Prospects and Limitations; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Cowie, B.W.; Byrne, M.J.; Witkowski, E.T.F. Small-scale insights into the above and below-ground invasion dynamics of Parthenium hysterophorus in a South African savanna: The potential role of stocking rate. S. Afr. J. Bot. 2022, 144, 229–237. [Google Scholar] [CrossRef]
- Putri, W.E. Allelopathic effect of Parthenium weed (Parthenium hysterophorus L.) leaf litter on the seedling emergence and growth of wheat (Triticum aestivum L.). IOP Conf. Ser. Earth Environ. Sci. 2022, 978, 012016. [Google Scholar] [CrossRef]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Kaur, M.; Yadav, A. Utility potential of Parthenium hysterophorus for its strategic management. Adv. Agric. 2014, 2014, 381859. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Mominul Islam, A.K.M.; Ahmad-Hamdani, M.S.; Hasan, M. Phytochemical constituents and allelopathic potential of Parthenium hysterophorus L. in comparison to commercial herbicides to control weeds. Plants 2021, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.; Rashid, H.U.; Amin, A.; Khan, I.A.; Shehzad, N. Allelopathic effect of Parthenium hysterophorus on germination and growth of some important crops and weeds of economic importance. Planta Daninha 2018, 36, e018176372. [Google Scholar] [CrossRef]
- Shi, B.; Dhileepan, K.; Adkins, S. The impact of Parthenium weed-amended substrates on the germination and early growth of a range of pasture and crop species. Agronomy 2021, 11, 1708. [Google Scholar] [CrossRef]
- Imad, M.; Idrees, M.; Hadi, F.; Memon, N.H.; Zhang, Z.Y. Allelopathic effect of Parthenium hysterophorus extract on seed germination and seedling growth of selected eight plants. Pak. J. Bot. 2021, 53, 187–2197. [Google Scholar] [CrossRef]
- Netsere, A.; Mendesil, E. Allelopathic effect of Parthenium hysterophorus L. aqueous extract on Soybean (Glycine max L.) and haricot bean (Phaseolus vulgaris L.) seed germination, shoot and root growth and dry matter production. J. Appl. Bot. Food Qual. 2011, 84, 219–222. [Google Scholar]
- Khosla, S.N.; Sobti, S.N. Effective control of Parthenium hysterophorus L. Pesticides 1981, 15, 18–19. [Google Scholar]
- Tamado, T. Biology and Management of Parthenium Weed (Parthenium hysterophorus L.) in Eastern Ethiopia. Ph.D. Thesis, Swedish University of Agricultural Sciences, Acta Universitatis Agriculturae Sueciae, Agraria 311, Uppsala, Sweden, 2002; 106p. [Google Scholar]
- Horo, J.; Gudisa, T.; Worku, E.; Tilahun, D. Distribution and abundance of Parthenium hysterophorus L. in Metekel Zone, Northwest Ethiopia. Am. J. Plant Sci. 2020, 11, 1101–1110. [Google Scholar] [CrossRef]
- Foxcroft, L.C. Alien Biota Section Records; Scientific Services Department, Unpublished Records; South African National Parks: Skukuza, South Africa, 2001. [Google Scholar]
- Witt, A.; Beale, T.; Van Wilgen, B.W. An assessment of the distribution and potential ecological impacts of invasive alien plant species in eastern Africa. Trans. R. Soc. S. Afr. 2018, 73, 217–236. [Google Scholar] [CrossRef]
- Patel, S. Harmful and beneficial aspects of Parthenium hysterophorus: An update. 3 Biotech 2011, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Khan, M.A. Adsorption studies on Parthenium hysterophorous weed: Removal and recovery of Cd (II) from wastewater. J. Hazard. Mater. 2006, 135, 242–248. [Google Scholar] [CrossRef]
- Akter, A.; Zuberi, M.I. Invasive alien species in Northern Bangladesh: Identification, inventory and impacts. Int. J. Biodivers. Conserv. 2009, 15, 129–134. [Google Scholar]
- Das, B.; Reddy, V.S.; Krishnaiah, M.; Sharma, A.V.S.; Ravi Kumar, K.; Rao, J.V.; Sridhar, V. Acetylated pseudoguaianolides from Parthenium hysterophorus and their cytotoxic activity. Phytochemistry 2007, 68, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Saxena, D.B. Pesticidal properties of parthenin (from Parthenium hysterophorus) and related compounds. Pest Manag. Sci. 2001, 57, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Vivekanand, V.; Ganguly, R.; Singh, R.P. Parthenium sp. as a plant biomass for the production of alkalitolerant xylanase from mutant Penicillium oxalicum SAUE-3.510 in submerged fermentation. Biomass Bionergy 2009, 33, 581–588. [Google Scholar] [CrossRef]
- Evans, H. Parthenium hysterophorus: A review of its weed status and the possibilities for biological control. Biocontrol News Inf. 1997, 18, 89–98. [Google Scholar]
- Gunaseelan, V.N. Parthenium as an additive with cattle manure in biogas production. Biol. Wastes. 1987, 21, 195–202. [Google Scholar] [CrossRef]
- Kishor, P.; Ghosh, A.K.; Singh, S.; Maury, B.R. Potential use of parthenium (Parthenium hysterophorus L.) in agriculture. Asian J. Agric. Res. 2010, 4, 220–225. [Google Scholar] [CrossRef]
- Javaid, A. Use of parthenium weed as green manure for maize and mungbean production. Philipp. Agric. Sci. 2008, 91, 478–482. [Google Scholar]
- Lata, H.; Garg, V.K.; Gupta, R.K. Sequestration of nickel from aqueous solution onto activated carbon prepared from Parthenium hysterophorus L. J. Hazard. Mater. 2008, 157, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Reid, A.M.; Sims-Chilton, N.M.; Buckley, Y.M.; Dhileepan, K.; Hastwell, G.T.; Nordblom, T.L.; Raghu, S. Review of approaches to evaluate the effectiveness of weed biological control agents. Biol. Control. 2009, 5, 1–15. [Google Scholar] [CrossRef]
- Maishi, A.I.; Ali, P.K.S.; Chaghtai, S.A.; Khan, G. A proving of Parthenium hysterophorus, L. Br Homeopath. J. 1998, 87, 17–21. [Google Scholar] [CrossRef]
- Lakshmi, C.; Srinivas, C.R. Parthenium: A wide angle view. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 296–306. [Google Scholar] [PubMed]
- Mane, J.D.; Jadav, S.J.; Ramaiah, N.A. Production of oxalic acid from dry powder of Parthenium hysterophorus L. J. Agric. Food Chem. 1986, 34, 989–990. [Google Scholar] [CrossRef]
- Pandey, D.K. Inhibition of Salvinia (Salvinia molesta Mitchell) by parthenium (Parthenium hysterophorus L.). I. Effect of leaf residue and allelochemicals. J. Chem. Biol. 1994, 19, 2651–2662. [Google Scholar] [CrossRef]
- Parashar, V.; Parashar, R.; Sharma, B.; Pandey, A. Parthenium leaf extract mediated synthesis of silver nano particles: A novel approach towards weed utilization. Dig. J. Nanomater. Biostructures 2009, 4, 45–50. [Google Scholar]
- Rajeshwari, S.; Subburam, V. Activated parthenium carbon as an adsorbent for the removal of dyes and heavy metal ions from aqueous solution. Bioresour. Technol. 2002, 85, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Surib-Fakim, A.; Swerab, M.D.; Gueho, J.; Dullo, E. Medicinal plants of Rodrigues. Int. J. Pharmacogn. 1996, 34, 2–14. [Google Scholar] [CrossRef]
- Venkataiah, B.; Ramesh, C.; Ravindranath, N.; Das, B. Charminarone, a seco-pseudoguaianolide from Parthenium hysterophorus. Phytochemistry 2003, 63, 383–386. [Google Scholar] [CrossRef]
- Holm, L.; Doll, J.; Holm, E.; Pancho, J.V.; Herberger, J.P. World Weeds: Natural Histories and Distribution; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Abdulkerim-Ute, J.; Legesse, B. Parthenium hysterophorus L: Distribution, impact, and possible mitigation measures in Ethiopia. Trop. Subtrop. Agroecosystems 2016, 19, 61–72. [Google Scholar] [CrossRef]
- Adkins, S.W.; McClay, A.; Bajwa, A.A. Biology and ecology. In Parthenium Weed: Biology, Ecology and Management; Adkins, S., Shabbir, A., Dhileepan, K., Eds.; Centre for Agriculture and Bioscience International: London, UK, 2018; pp. 7–39. [Google Scholar]
- Nigatu, L.; Hassen, A.; Sharma, J.; Adkins, S. Impact of Parthenium hysterophorus on grazing land communities in north-eastern Ethiopia. Weed Biol. Manag. 2010, 10, 143–152. [Google Scholar] [CrossRef]
- Niguse, H.; Kifle, B. Socio-economic impacts of Parthenium hysterophorus L. in east Shewa and West Arsi zones of Ethiopia. Int. J. Agric. Res. Innov. Technol. 2016, 6, 5–11. [Google Scholar]
- Ojija, F.; Arnold, S.E.J.; Treydte, A.C. Impacts of alien invasive Parthenium hysterophorus on flower visitation by insects to co-flowering plants. Arthropod-Plant Interact. 2019, 13, 719–734. [Google Scholar] [CrossRef]
- Bufebo, B.; Elias, E. Distribution and socio-economic impacts of invasive alien plant species in Ethiopia: A Review. Open J. Plant. Sci. 2018, 3, 026–033. [Google Scholar]
- Dhileepan, K. Biological control of Parthenium (Parthenium hysterophorus) in Australian rangeland translates to improved grass production. Weed Sci. 2007, 55, 497–501. [Google Scholar] [CrossRef]
- Shabbir, A.; Bajwa, R. Distribution of parthenium weed (Parthenium hysterophorus L.): An alien invasive weed species threatening the biodiversity of Islamabad. Weed Biol. Manag. 2006, 6, 89–95. [Google Scholar] [CrossRef]
- Narasimhan, T.R.; Ananth, M.; Swamy, M.N.; Babu, M.R.; Mangala, A.; Rao, P.V.S. Toxicity of Parthenium hysterophorus L. to cattle and buffaloes. Experientia 1977, 33, 1358–1359. [Google Scholar] [CrossRef] [PubMed]
- Tudor, G.; Ford, A.L.; Armstrong, T.; Bromage, E. Taints in meat from sheep grazing Parthenium hysterophorus. Anim. Prod. Sci. 1982, 22, 43–46. [Google Scholar] [CrossRef]
- TNAU. Parthenium on Livestock Health. 2016. Available online: http://agritech.tnau.ac.in/agriculture/agri_weedmgt_parthenium_livestock.html (accessed on 16 February 2024).
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Saxena, D.B.; Arora, V. Effect of pathenin-A sesquiterpene lactone from Parthenium hysterophorus L. on early growth and physiology of Ageratum conyzoides. J. Chem. Ecol. 2002, 28, 2169–2179. [Google Scholar] [CrossRef]
- Kaur, L.; Malhi, D.S.; Cooper, R.; Kaur, M.; Sohal, H.S.; Mutreja, V.; Sharma, A. Comprehensive review on ethnobotanical uses, phytochemistry, biological potential and toxicology of Parthenium hysterophorus L.: A journey from noxious weed to a therapeutic medicinal plant. J. Ethnopharmacol. 2021, 281, 114525. [Google Scholar] [CrossRef] [PubMed]
- Novoa, A.; Dehnen-Schmutz, K.; Fried, J.; Vimercati, G. Does public awareness increase support for invasive species management? Promising evidence across taxa and landscape types. Biol. Invasions 2017, 19, 3691–3705. [Google Scholar] [CrossRef]
- Belgeri, A.; Bajwa, A.A.; Shabbir, A.; Navie, S.; Vivian-Smith, G.; Adkins, S. Managing an invasive weed species, Parthenium hysterophorus, with suppressive plant species in Australian Grasslands. Plants 2020, 9, 1587. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Pawar, D.; Gharde, Y.; Kumar, S.; Dhagat, S. Integrated Management of Parthenium; ICAR-DWR: Jabalpur, India, 2023; Volume 68, pp. 1–6. [Google Scholar]
- Mao, R.; Bajwa, A.A.; Adkins, S. A superweed in the making: Adaptations of Parthenium hysterophorus to a changing climate. A review. Agron. Sustain. Dev. 2021, 41, 47. [Google Scholar] [CrossRef]
- Taye, T.; Gossmann, M.; Einhorn, G.; Büttner, C.; Metz, R.; Abate, D. The potential of pathogens as biological control of parthenium weed (Parthenium hysterophorus L.) in Ethiopia. Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2002, 67, 409–420. [Google Scholar]
- Wahab, S. Management of Parthenium through an integrated approach initiatives, achievements and research opportunities in India. In Proceedings of the 2nd International Conference on Parthenium Management, University of Agricultural Sciences, Bangalore, India, 5–7 December 2005; pp. 36–43. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).