Abstract
The invasive golden apple snail Pomacea canaliculata has a strong reproductive capacity and has rapidly spread in Asian countries. Current control methods include physical, chemical, and biological approaches, but there has been limited research on the control of P. canaliculata in its different life stages. This study assessed the effectiveness of using giant river prawns Macrobrachium rosenbergii in controlling juveniles of P. canaliculata through a controlled indoor experiment. The density, size, and dispersal range of recently hatched juvenile snails were significantly lower among those kept with prawns than those kept without prawns, indicating a control effect of M. rosenbergii at least on P. canaliculata juveniles. Furthermore, the study speculates on the potential application of M. rosenbergii in the context of a rice–prawn symbiotic system of ecological farming to control invasive P. canaliculata. In terms of effectiveness and safety, its application might lead to a win-win situation for both rice-farm profits and the ecological benefits of invasive species control.
1. Introduction
The golden apple snail Pomacea canaliculate (family Ampullariidae in the order Mesogastropoda) is native to tropical and subtropical regions of South America, including the Plata and Amazon river basins [1,2]. Introduced in China in the 1980s for edible snail farming, the effort was later abandoned because of the snail’s poor taste [1,2,3]. However, owing to the species’ strong reproductive capacity and adaptability, P. canaliculate quickly spread to freshwater environments, such as rice paddies and water chestnut fields [1,3,4,5], leading to explosive invasions in multiple provinces in China, Vietnam, the Philippines and Thailand [6,7,8]. Its rapid population growth and extensive plant consumption have caused wide-ranging impacts on local biodiversity through competition with native species [6], and the species has been listed among the top 100 of the world’s worst invasive alien species [9]. New species of Pomacea are continuously being identified using molecular methods; thus, apart from population expansions, the genus Pomacea is relatively speciose [10].
Currently, the control methods for P. canaliculate mainly include physical, chemical, and biological methods [4]. Physical control involves picking up, trapping, or intercepting snails and their eggs. This process is simple and environmentally friendly but requires substantial manpower and materials with associated financial costs and shows slow results [6,11]. Chemical control is the most commonly used method, employing molluscicides such as formulations of niclosamide, triclosan, and piperonyl butoxide [12]. This approach has several advantages, such as quick effect, broad applicability, and an evident control effect, but it also incurs high costs and poses potential toxicity to aquatic organisms, eventually affecting ecosystem functionality [13]. Some researchers have explored molluscicides derived from plants to attract and kill P. canaliculate, such as extracts from the perennial plant Ipomoea cairica [14,15,16,17]. However, the mechanism of action of plant-based molluscicides is not well studied, and the effective molluscicidal components are difficult to identify and quantify [16,18,19].
Biological control, which utilizes inter-species interactions, offers advantages such as durability, high efficiency, environmental friendliness, and relatively low costs [20], making this an innovative approach for Pomacea management [21,22]. One such method is to control the abundance of vegetation that species of Pomacea prefer, which has been proven effective [23]. Furthermore, researchers found that 26 out of 46 freshwater animals investigated were capable of preying on P. canaliculate juveniles [24]. Currently, bio-control of various ampullariid species has employed the common shelducks Tadorna tadorna [24,25], Chinese soft-shelled turtle Pelodiscus sinensis [22], common carp Cyprinus carpio [26], and black carp Mylopharyngodon piceus [26], with relatively good control effects. Nevertheless, the life cycle of P. canaliculate consists of three stages: eggs, juveniles, and adults, with a total lifespan of 2–5 years [27]; with high egg-production rates, one female snail can reproduce up to 300,000 juveniles annually, and the juveniles can reach sexual maturity within 3–4 months, leading to overlapping generations [28]. Most existing biological control research on these snails has focused on the clearance and control of adults while neglecting their extremely strong reproductive ability, which results in rapid population recovery [21,22,24,25,29]. Therefore, the entire life cycle of P. canaliculate juveniles needs to be considered part of the control effort, but there are few reports on the biological control of juvenile snails.
Based on its rapid growth and development, a broad diet, large size, and delicious meat, the Malaysian freshwater prawn Macrobrachium rosenbergii (family Palaemonidae in the order Decapoda) has become an important species in Asian shrimp aquaculture industry, supporting agricultural development in many regions [30,31]. Introduced in China in 1976, the artificial rearing of prawn larvae became successful in subsequent years, leading to widespread farming across provinces and cities [32]. Although the red swamp crayfish Procambarus clarkii has been the main species used in the traditional rice–prawn symbiotic system of ecological farming [33,34], in recent years, factors such as degraded germplasm quality, insufficient seed supply, and weak industry systems have affected farming profits. Instead, M. rosenbergii has gradually become a new option in this model of ecological farming owing to its rapid growth, short farming cycle, stable market price, and suitability for rearing during high temperatures in summer [35,36].
In Bangladesh and China, some animal–rice farmers now culture snails for use as feed for prawn [37]; furthermore, snail shells in the diet of juvenile prawns maybe beneficial for their growth [38]. Considering the characteristics of P. canaliculate invasions and the current trend to promote and develop rice–prawn symbiotic ecological farming techniques as well as the suitability of M. rosenbergii growth and farming in regions known to have these snail invasions [36,39,40], this study investigated the effect of M. rosenbergii as a predator on the survival and growth of P. canaliculate juveniles under controlled laboratory conditions, thereby exploring the potential to use this prawn species to control the snails in rice paddies.
2. Materials and Methods
2.1. Experimental Materials
Pomacea canaliculata eggs and subadults were collected from rice fields and pond aquaculture systems in Maoming, Guangdong, China. For the egg collection, freshly laid egg masses were chosen from the same day’s oviposition. For the collection of subadults, individuals with a shell height of 2–3 cm were selected. Once a sufficient quantity of snail egg masses and subadults were collected, they were transported to the laboratory for use in the experiment.
Prior to the experiment, the collected subadult snails were acclimated to the laboratory environment by housing them in plastic tanks measuring 0.6 m × 0.4 m × 0.4 m (length × width × height) for 3 days. The tanks were covered with fine wire mesh to prevent the snails from escaping. The snails were fed fresh lettuce as food once daily and provided with dechlorinated, aerated water, with the water periodically replaced to maintain the cleanliness of the tanks. Following acclimation, individuals demonstrating good vitality were selected for the experiment.
Juvenile M. rosenbergii for the study were sourced from Jiangsu Shufeng Prawn Breeding Co. Ltd, Gaoyou, China, and then reared in experimental aquaculture ponds at Huzhou University. Individuals displaying normal vitality and consistent size (~8 cm in total length) were chosen for the experiment.
2.2. Experimental Design
The experimental design of the indoor trials consisted of two snail size treatments (subadult snails vs. recently hatched juvenile snails) × two shrimp treatments (snail with shrimp vs. snail without shrimps) × 3 replicates and a snail-absent control × 3 replicates. Four treatment groups plus the control group were established as follows:
- Subadult snails reared with shrimps: Each experimental tank was stocked with 40 P. canaliculate subadults (shell width of ~2 cm) along with 8 M. rosenbergii; to mitigate against damage caused by aggressive interactions, only female shrimps were selected;
- Subadult snails reared without shrimps: Each experimental tank was stocked with 40 P. canaliculate subadults (shell width of ~2 cm) and without the addition of M. rosenbergii;
- Recently hatched juvenile snails reared with shrimps: 10 egg masses (~20 g each) of P. canaliculate were evenly distributed on a wire mesh (2 mm aperture, which allowed newly hatched juveniles to pass through but prevented the passage of subadults) positioned at the top of each tank, and tank was stocked with 8 M. rosenbergii;
- Recently hatched juvenile snails reared without shrimp: Similar to the above treatment, 10 egg masses (~20 g each) of P. canaliculate were evenly distributed on the wire mesh on the top of each tank but without the presence of M. rosenbergii;
- Snail-absent control with only shrimps: The experimental tank contained only 8 M. rosenbergii.
Each treatment was replicated three times, resulting in a total of 15 experimental tanks (0.6 m length × 0.4 m width× 0.4 m height).
The experimental tanks were supplied with air tubes for continuous aeration. Specialized pellets formulated for M. rosenbergii, based on their daily feeding requirements in routine aquaculture, were added to the tanks. Additionally, an ample quantity of fresh lettuce was provided to nourish the subadult snails. Each evening, any remaining food residue was cleared from the tanks.
2.3. Data Collection and Analysis
To measure snail population density, three random areas of 10 × 10 cm were selected in each tank to count the number of snails. To measure snail size, randomly selected snails from each experimental tanks were measured for shell width using a vernier caliper. To calculate the distribution probability of juvenile and subadult snails in the experimental tanks, the bottom of each tank was divided into 24 areas of 10 × 10 cm each, and the number of areas with snails present was counted, which was divided by 24, thereby assessing the impact of M. rosenbergii on the extent of area where snails were active.
The egg masses began hatching on day 11 of the experiment and finished hatching by day 18. The population density of juvenile snails was measured across 6 days of the experiment, from day 12 to day 17. Snail shell width was measured on days 12, 14, and 17. The distribution probability of snails was also measured for 6 days, from day 12 to day 17.
The body length of M. rosenbergii was determined by briefly removing each prawn from the tank and measuring it with vernier calipers.
To establish the treatment with subadult snails, synchronization with the treatment groups with egg masses was maintained by introducing subadult snail into the experimental tanks on day 11, which corresponded to the start of egg hatching. The population density of subadult snails was measured for 6 days, on days 12 to 17. Shell width of subadult snails was measured on the 12th, 14th, and 17th days. The distribution probability of subadult snails in the experimental tanks was also measured for 6 days, from days 12 to 17. The body length of M. rosenbergii was measured on days 1, 12, and 18 of the experiment.
Two-way analysis of variance and t-tests were employed to assess differences between treatments with and without M. rosenbergii as well as differences between treatments with the two snail sizes. The data were analyzed using STATISTICA 13, and graphical representations were created using Adobe Illustrator.
3. Results
3.1. Impact of M. rosenbergii on the Quantity of P. canaliculata
The density of newly hatched juvenile snails was significantly lower in the presence of shrimp (“shrimp-present” group) than in the absence of shrimp (“shrimp-absent” group) (Figure 1). The mean density of recently hatched juvenile snails in the shrimp-present group was 3.15 ± 0.89 ind./dm², whereas in the shrimp-absent group, it was 29.31 ± 2.60 ind./dm², and the difference was significant (t = 9.519, p < 0.001).
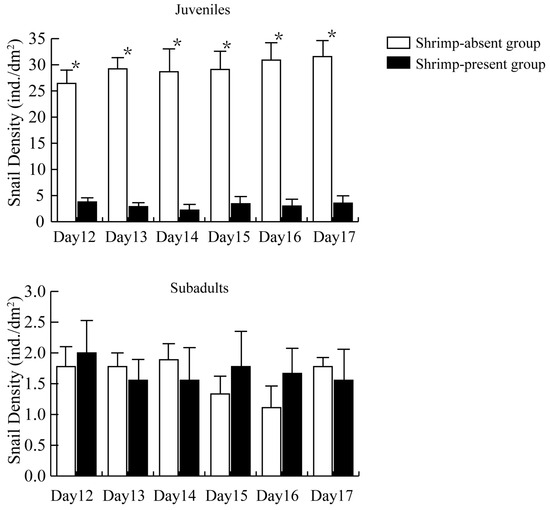
Figure 1.
The density of recently hatched juvenile snails versus subadult snails in the shrimp-present and shrimp-absent groups. The asterisk (*) represents a significant difference (p < 0.05) between shrimp-absent group and shrimp-present group.
As the days since hatching progressed, the density of recently hatched juvenile snails in the shrimp-absent group exhibited a slightly increasing trend, although differences across the 6 days measured were not significant (p > 0.05). In contrast, the density of recently hatched juvenile snails in the shrimp-present group remained consistently low across the days measured.
The density of subadult snails showed no significant differences across days of the experiment in the shrimp-present group as compared with in the shrimp-absent group (Figure 1), with the former group largely maintaining the initial density of subadults set in the experiment.
Macrobrachium rosenbergii can directly ingest smaller juvenile snails, whereas they employ their chelae to shred the shells of larger juveniles before ingesting them. Subadult snail shells that had been damaged from attacks by M. rosenbergii were also noted throughout the experiment.
3.2. Size of P. canaliculata Surviving in the Presence of M. rosenbergii
Beyond the first observation, the shell width of recently hatched juvenile snails was significantly smaller in the shrimp-present group than in the shrimp-absent group (Figure 2). The average shell height of juvenile snails in the shrimp-present group was 0.036 ± 0.006 cm over 6 days, whereas it was 0.114 ± 0.008 cm in the shrimp-absent group, showing a significant difference between the two groups (t = 8.082, p < 0.001).
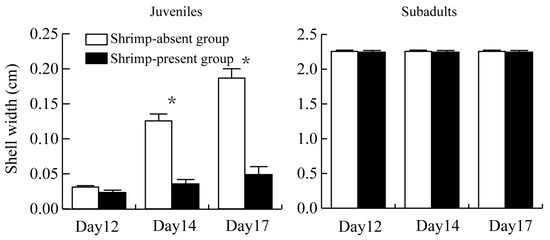
Figure 2.
Shell width of recently hatched juvenile snails versus subadult snails in the shrimp-present and shrimp-absent groups. The asterisk (*) represents a significant difference (p < 0.05) between shrimp-absent group and shrimp-present group.
Shell width of recently hatched juvenile snails increased significantly (p < 0.001) as days since hatching increased in both the shrimp-present and shrimp-absent groups, but width growth was greater in the shrimp-absent group.
Shell width of subadult snails did not differ significantly between the shrimp-present and shrimp-absent groups, and there was minimal change in shell width observed over the 6-day observation period.
3.3. Influence of M. rosenbergii on the Activity of P. canaliculata
The distribution probability for recently hatched juvenile snails was significantly lower in the shrimp-present group than in the shrimp-absent group (Figure 3). The average distribution probability of juvenile snails in the shrimp-present group over the 6-day observation period was 0.106 ± 0.016, while in the shrimp-absent group, it was 0.899 ± 0.007, and the difference between groups was significant (t = 47.032, p < 0.001).
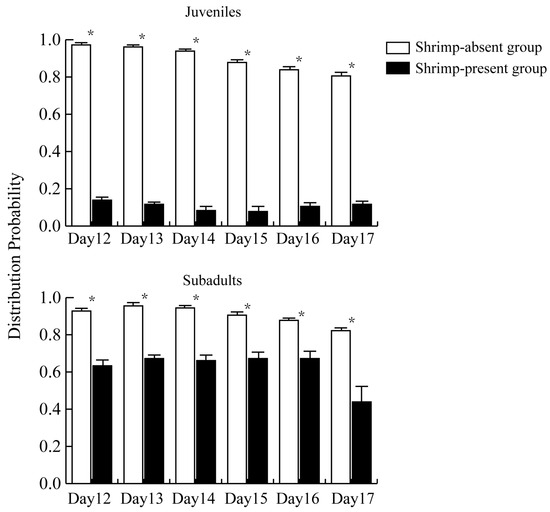
Figure 3.
Distribution probability for recently hatched juvenile snails and subadult snails in the shrimp-present and shrimp-absent groups. The asterisk (*) represents a significant difference (p < 0.05) between shrimp-absent group and shrimp-present group.
As the days since hatching increased, the distribution probability for newly hatched juvenile snails gradually decreased in the shrimp-absent group (p < 0.05), indicating a trend of gradual aggregation. The distribution probability for recently hatched juvenile in the shrimp-present group remained consistently low, with no clear pattern observed across the days of the experiment. Recently hatched juveniles in the shrimp-absent group were observed to beg more evenly distributed in the experimental tank, while the juveniles in the shrimp-present group were concentrated mainly at the edges.
Overall, the distribution probability for subadult snails was likewise significantly lower in the shrimp-present group than in the shrimp-absent group (Figure 3). The average distribution probability for subadult snails over six days was 0.625 ± 0.018 in the shrimp-present group and 0.906 ± 0.006 in the shrimp-absent group, revealing a significant difference between the two groups (t = 14.851, p < 0.001).
The Tukey HSD test results indicated no significant difference in the average distribution probability between the shrimp-absent groups of recently hatched juvenile and subadult snails (p = 0.984). However, there was a significant difference in the average distribution probability between shrimp-present groups of hatched juveniles and subadult (p < 0.001).
3.4. M. rosenbergii Survival and Size Changes and Estimation of their Control Efficiency on P. canaliculata
A comparison of the change in size of the M. rosenbergii among the treatment groups and the controls (Table 1) showed no significant differences during the experiment, and all prawns survived. Therefore, variations in the individual sizes of the M. rosenbergii throughout the experiment could not have influenced the P. canaliculata that they were reared with. The M. rosenbergii exhibited slight growth during the 18-day experiment, with no significant differences in growth among groups, and the magnitude of size change was minor.

Table 1.
The change in size of Macrobrachium rosenbergii during the rearing experiment with Pomacea canaliculata.
Based on the results, a preliminary assessment of the control efficiency of M. rosenbergii on P. canaliculata could be made. Accordingly, the control efficiency of M. rosenbergii based on the population density of newly hatched juveniles of P. canaliculata was estimated to be 89.26%, with an inhibitory effect on juveniles’ growth based on the shell width of surviving snails reaching 68.61%, and inhibition of the extent of area where juvenile snails were active was estimated as 88.16% (Table 2). However, M. rosenbergii had no significant impact on the density and growth of subadults of P. canaliculata (p > 0.05), and inhibition of the active area of the subadult reached 30.98% (Table 2).

Table 2.
Control efficiency * of Macrobrachium rosenbergii on Pomacea canaliculata in the laboratory experiment, based on snail density (number), snail growth (shell width), and extent of the snails’ active area.
4. Discussion
4.1. Effectiveness of M. rosenbergii in Controlling P. canaliculata
The results of this experiment demonstrate that M. rosenbergii will prey on newly hatched juveniles of P. canaliculata, significantly repressing the population of juvenile snail snails (Figure 1). Similarly, Roberts et al. [41] found that M. rosenbergii will prey on the freshwater snail Biomphalaria glabrata.
The presence of M. rosenbergii slowed the growth of individual P. canaliculata juveniles in the experimental tanks (Figure 2). This could be attributed to reduced opportunities for the juvenile snails to feed because of the presence of predation by the prawns. Differential feeding preferences exhibited by M. rosenbergii for various sizes of snails results in them selecting juvenile snails [42].
Additionally, the presence of M. rosenbergii limited the distribution of P. canaliculata juveniles in the experimental tanks (Figure 3). The consumption of juvenile snails by M. rosenbergii would accordingly result in less area occupied by surviving snails [43]. However, juvenile snails might exhibit active avoidance behavior, but this requires further investigation. These findings demonstrate the potential value of using M. rosenbergii in controlling invasive P. canaliculata, managing both the scale of P. canaliculata reproduction and the spread of juveniles.
Notably, in our experiment, M. rosenbergii had almost no impact on subadults of P. canaliculata with a shell height greater than 2 cm (Table 2). Thus, it can be predicted that the inhibitory effect of M. rosenbergii will be limited for populations of larger-sized adults. This result is partly attributable to the small size of M. rosenbergii used in the experiment and partly because the shells of subadult or adult P. canaliculata are relatively hard, making it difficult for many predators to break them [21,44,45]. Therefore, when using cultured M. rosenbergii for controlling P. canaliculata invasion, additional measures like manual removal of adult snails may be needed to improve the control effect.
4.2. Potential Application of M. rosenbergii for Controlling P. canaliculata Invasion
Currently, some studies have conducted experiments on controlling P. canaliculata using symbiotic systems of ecological farming, such as rice–duck and rice–fish schemes, primarily by employing natural predators of the invasive species [46,47]. Studies that utilized the ecological farming model of raising Tadorna tadorna in rice fields to control P. canaliculata found that co-cultivating ducks with rice could also reduce the overwintering and residual snail population in rice fields [48,49]. The co-cultivation of ducks primarily takes advantage of their preference for feeding on juvenile snails, thereby reducing the snail population. However, during the early stages of plant growth, ducks may consume tender plant shoots, necessitating their timely removal from the rice paddies [50]. Moreover, the high cost and extensive labor required for duck farming, along with the need to strictly control the number of ducks released to avoid water pollution [51], limits the applicability of using rice–duck co-cultivation to control agricultural P. canaliculata.
Black carp are carnivorous fish that feed on snail meat. Researchers have found that stocking black carp can be an effective method for controlling P. canaliculata in water chestnut fields. However, to achieve better control of P. canaliculata, larger-sized black carp and a sufficient water depth that allows their movement are required [52], which imposes significant limitations on the widespread promotion of black carp for controlling agricultural invasions of P. canaliculata.
In contrast, M. rosenbergii can be farmed in a wide range of areas and has modest feed requirements, making it suitable for aquaculture. Moreover, P. canaliculata provides a rich and convenient source of biological material for M. rosenbergii to effectively prey on as a dietary supplement beyond mere feed, thereby achieving biocontrol. A previous study found that a higher percentage of P. canaliculata in the feed formula was more beneficial for the molting and growth of M. rosenbergii [53].
4.3. Potential Risks of Using M. rosenbergii for Biocontrol of P. canaliculata
Although some natural predators can exert some control over P. canaliculata, large-scale promotion of these predators poses certain biosecurity risks. Regarding the red swamp crayfish Procambarus clarkii, for example, its omnivory threatens native biodiversity, and its burrowing behavior can disrupt eco-structural integrity, with risks for using it as a bio-control agent [54]. In comparison, the use of M. rosenbergii to control P. canaliculata poses a lower safety risk. First, although M. rosenbergii is also an introduced species in China, it is a tropical shrimp with a preferred temperature range of 25–30 °C and a minimum limit of 14 °C [55]. Below 18 °C, it exhibits reduced activity, decreased feeding, slowed growth, and increased mortality, meaning it is unable to survive the winter in most parts of China and Japan [55,56]. Second, M. rosenbergii requires seawater for breeding, as it cannot complete its life history and reproduction in freshwater [32,55]. Therefore, it is difficult to establish a reproductive population in pure freshwater environments where P. canaliculata is active, making invasion virtually impossible. Furthermore, M. rosenbergii is a benthic species capable of swimming only short distances, making it unable to disperse over great distances [55,56]. Recent experiments have employed all-male prawn juveniles for biocontrol purposes, thereby mitigating the risks of its reproduction [42]. Thus, it appears that the use of M. rosenbergii, especially its sex-biased seeding [42], to control P. canaliculata invasion would have minimal impact on local biodiversity.
Currently, the deployment of M. rosenbergii for controlling invasive P. canaliculata in Asian rice paddies appears to be a prudent approach. However, considering the varying potential risks across different regions in Asia, separate strategies for its application should be considered. In Southeast Asia, where M. rosenbergii is a native species that does not pose a risk of biological invasion, its promotion for controlling P. canaliculata in rice paddy systems is highly feasible. However, in China and Japan, where M. rosenbergii is an introduced aquaculture species, there is a risk of its population expansion. Although the current capacity of M. rosenbergii for wild reproduction and dissemination is limited in these regions, precautionary measures against potential invasiveness are necessary when advocating its utilization for P. canaliculata control.
We recommend the following measures when considering M. rosenbergii for biocontrol:
- Consider its deployment in isolated water bodies, artificial wetlands, or rice paddies, avoiding introduction into natural water habitats;
- Employ physiologically stable M. rosenbergii juveniles for release to minimize the introduction of individuals with high variability. The utilization of sex-biased seedings of M. rosenbergii might to ensure biological security. Simultaneously, the potential for hybridization between M. rosenbergii and indigenous freshwater prawn species should also be investigated to prevent hybridization-induced population dispersion [57];
- Capture M. rosenbergii from the release areas during autumn and winter, subjecting them to dry ponds or sun-drying treatments.
Simultaneously, in East Asian countries in general, the use of locally adapted shrimp species with similar physiological traits and dietary habits to manage P. canaliculata reproduction and expansion is also worth investigating to further mitigate ecological risks associated with the use of a non-native species. For instance, in China, the indigenous East Asian river prawn M. nipponense shares similarities with M. rosenbergii and therefore merits research into its feasibility and effectiveness as a biocontrol agent against P. canaliculata.
5. Conclusions
This study demonstrated the moderate biocontrol effect of M. rosenbergii on P. canaliculata juveniles under laboratory conditions. Subsequent research could be conducted on a pilot scale, such as in rice fields or constructed wetland ecosystems, to further elucidate the value of using M. rosenbergii to control P. canaliculata. Given the uncertainties in profit of P. clarkii caused by fluctuations in the market price, seasonal diseases, and ecological risks for rice paddy farming as well as in rice–fish and rice–shrimp co-cultivation, introducing M. rosenbergii into fields affected by a rampant P. canaliculata invasion might be an effective and safe choice. On one hand, such application could optimize an aquaculture species and significantly improve economic returns in agricultural fields; however, the choice could help control these highly invasive snails, inhibiting their reproduction and spread, thus effectively reducing their harmful impact on agricultural fields, constructed wetlands, and other ecosystems, thereby producing positive ecological benefits.
Author Contributions
Conceptualization, Q.S. and H.L.; data curation, Y.W., Y.Z. and H.L.; formal analysis, Q.S.; methodology, Y.W., Y.Z. and H.L.; project administration, Q.S. and H.L.; writing—original draft, Y.W., H.L. and Q.S.; writing—review and editing, Q.S. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zhejiang Provincial Basic Public Welfare Research Program, grant number LGN20C190001, and the APC was funded by the National Wetland Museum of China Grant.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of National Wetland Museum of China (protocol code 20190112, Jan,2019).
Data Availability Statement
All data, models, or code generated or used during the study are available from the corresponding author by request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayes, K.A.; Joshi, R.C.; Thiengo, S.C.; Cowie, R.H. Out of South America: Multiple Origins of Non-native Apple Snails in Asia. Divers. Distrib. 2008, 14, 701–712. [Google Scholar] [CrossRef]
- Joshi, R.C.; Parera, X.V. The Rice Apple Snail in Spain: A Review. Int. Pest Control 2017, 59, 106. [Google Scholar]
- Horgan, F.G.; Stuart, A.M.; Kudavidanage, E.P. Impact of Invasive Apple Snails on the Functioning and Services of Natural and Managed Wetlands. Acta Oecologica 2014, 54, 90–100. [Google Scholar] [CrossRef]
- De Brito, F.C.; Joshi, R.C. The Golden Apple Snail Pomacea Canaliculata: A Review on Invasion, Dispersion and Control. Outlooks Pest Manage. 2016, 27, 157–163. [Google Scholar] [CrossRef]
- Byers, J.E.; Mcdowell, W.G.; Dodd, S.R.; Haynie, R.S.; Pintor, L.M.; Wilde, S.B. Climate and pH Predict the Potential Range of the Invasive Apple Snail (Pomacea insularum) in the Southeastern United States. PLoS ONE 2013, 8, e56812. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, Q.; Xu, Y. Golden Apple Snails. In Biological Invasions and Its Management in China: Volume 2; Wan, F., Jiang, M., Zhan, A., Eds.; Springer: Singapore, Singapore, 2017; pp. 33–47. ISBN 978-981-10-3427-5. [Google Scholar]
- Nghiem, L.T.P.; Soliman, T.; Yeo, D.C.J.; Tan, H.T.W.; Evans, T.A.; Mumford, J.D.; Keller, R.P.; Baker, R.H.A.; Corlett, R.T.; Carrasco, L.R. Economic and Environmental Impacts of Harmful Non-Indigenous Species in Southeast Asia. PLoS ONE 2013, 8, e71255. [Google Scholar] [CrossRef]
- Dumidae, A.; Janthu, P.; Subkrasae, C.; Polseela, R.; Mangkit, B.; Thanwisai, A.; Vitta, A. Population Genetics Analysis of a Pomacea Snail (Gastropoda: Ampullariidae) in Thailand and its Low Infection by Angiostrongylus cantonensis. Zool. Stud. 2021, 60, e31. [Google Scholar] [CrossRef]
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the World’s Worst Invasive Alien Species. Biol. Invasions. 2014, 16, 981–985. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, X. A New Species of Apple Snail in the Genus Pomacea (Gastropoda: Caenogastropoda: Ampullariidae). Zool. Stud. 2019, 58, e13. [Google Scholar] [CrossRef]
- Yahaya, H.; Badrulhadza, A.; Sivapragasam, A.; Nordin, M.; Muhamad Hisham, M.N.; Mirudin, H. Invasive apple snails in Malaysia. In Biology and Management of Invasive Apple Snails, 2nd ed.; Joshi, R.C., Cowie, R.H., Sebastian, L.S., Eds.; Philippine Rice Research Institute (PhilRice): Nueva Ecija, Philippine, 2017; pp. 169–195. [Google Scholar]
- Xu, Y.; Li, A.J.; Li, K.; Qin, J.; Li, H. Effects of Glyphosate-based Herbicides on Survival, Development and Growth of Invasive Snail (Pomacea canaliculata). Aquat. Toxicol. 2017, 193, 136–143. [Google Scholar] [CrossRef]
- Duong, H.V.; Nguyen, T.C.; Nguyen, X.T.; Nguyen, M.Q.; Nguyen, P.H.; Vo, T.T. Evaluating the Presence of Pesticide Residues in Organic Rice Production in An Giang Province, Vietnam. J. Sustain. Develop. 2022, 15, 49. [Google Scholar] [CrossRef]
- Plan, M.R.R.; Saska, I.; Cagauan, A.G.; Craik, D.J. Backbone Cyclised Peptides from Plants Show Molluscicidal Activity against the Rice Pest Pomacea canaliculata (Golden Apple Snail). J. Agric. Food. Chem. 2008, 56, 5237–5241. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, W.; Dong, X.; Hu, R.; Nan, X. Molluscicidal Activity of Cardiac Glycosides from Nerium indicum Against Pomacea canaliculata and Its Implications for the Mechanisms of Toxicity. Environ. Toxicol. Pharmacol. 2011, 32, 226–232. [Google Scholar] [CrossRef]
- Ding, W.; Huang, R.; Zhou, Z.; He, H.; Li, Y. Ambrosia artemisiifolia as a Potential Resource for Management of Golden Apple Snails, Pomacea canaliculata (Lamarck). Pest Manag. Sci. 2018, 74, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.C.D.; Gosmann, G.; Oliveira, G.T. Extracts of the Unripe Fruit of Ilex paraguariensis as a Potential Chemical Control against the Golden Apple Snail Pomacea canaliculata (Gastropoda, Ampullariidae). Nat. Prod. Res. 2019, 33, 2379–2382. [Google Scholar] [CrossRef] [PubMed]
- Noorshilawati, A.A.; Suraya, A.N.; Rossiyah, S.S. Molluscicidal Activity of Ipomoea batatas Leaf Extracts against Pomacea canaliculata (Golden Apple Snail). Food Res. 2020, 4, 131–137. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, S.; Zeng, J.; Chen, Y.; Guo, Y.; Liu, J.; He, A. Molluscicidal Activity of Nicotiana tabacum Extracts on the Invasive Snail Pomacea canaliculata. Sci. Rep. 2023, 13, 11597. [Google Scholar] [CrossRef]
- Culliney, T.W. Benefits of Classical Biological Control for Managing Invasive Plants. Crit. Rev. Plant Sci. 2005, 24, 131–150. [Google Scholar] [CrossRef]
- Azmi, W.A.; Khoo, S.C.; Ng, L.C.; Baharuddin, N.; Aziz, A.A.; Ma, N.L. The Current Trend in Biological Control Approaches in the Mitigation of Golden Apple Snail Pomacea spp. Biol. Control. 2022, 175, 105060. [Google Scholar] [CrossRef]
- Dong, S.; Zheng, G.; Yu, X.; Fu, C. Biological Control of Golden Apple Snail, Pomacea canaliculata by Chinese Soft-shelled Turtle, Pelodiscus sinensis in the Wild Rice, Zizania latifolia Field. Sci. Agric. 2012, 69, 142–146. [Google Scholar] [CrossRef]
- Yam, R.; Fan, Y.; Wang, T. Importance of Macrophyte Quality in Determining Life-History Traits of the Apple Snails Pomacea canaliculata: Implications for Bottom-Up Management of an Invasive Herbivorous Pest in Constructed Wetlands. Int. J. Environ. Res. Public Health 2016, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Yusa, Y.; Sugiura, N.; Wada, T. Predatory Potential of Freshwater Animals on an Invasive Agricultural Pest, the Apple Snail Pomacea canaliculata (Gastropoda: Ampullariidae), in Southern Japan. Biol. Invasions 2006, 8, 137–147. [Google Scholar] [CrossRef]
- Horgan, F.G. Ecology and Management of Apple Snails in Rice. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 393–417. ISBN 978-3-319-47516-5. [Google Scholar]
- Ip, K.K.L.; Liang, Y.; Lin, L.; Wu, H.; Xue, J.; Qiu, J. Biological Control of Invasive Apple Snails by Two Species of Carp: Effects on Non-target Species Matter. Biol. Control 2014, 71, 16–22. [Google Scholar] [CrossRef]
- Gilioli, G.; Pasquali, S.; Martín, P.R.; Carlsson, N.; Mariani, L. A Temperature-dependent Physiologically Based Model for the Invasive Apple Snail Pomacea canaliculata. Int. J. Biometeorol. 2017, 61, 1899–1911. [Google Scholar] [CrossRef]
- Constantine, K.L.; Makale, F.; Mugambi, I.; Chacha, D.; Rware, H.; Muvea, A.; Kipngetich, V.K.; Tambo, J.; Ogunmodede, A.; Djeddour, D.; et al. Assessment of the Socio-economic Impacts Associated with the Arrival of Apple Snail (Pomacea canaliculata) in Mwea Irrigation Scheme, Kenya. Pest Manag. Sci. 2023. [Google Scholar] [CrossRef]
- Bertolero, A.; Navarro, J. A Native Bird as a Predator for the Invasive Apple Snail, a Novel Rice Field Invader in Europe. Aquatic Conserv: Mar Freshw Ecosyst. 2018, 28, 1099–1104. [Google Scholar] [CrossRef]
- Tan, K.; Wang, W. The Early Life Culture and Gonadal Development of Giant Freshwater Prawn, Macrobrachium rosenbergii: A Review. Aquaculture 2022, 559, 738357. [Google Scholar] [CrossRef]
- Pillai, B.R.; Ponzoni, R.W.; Das Mahapatra, K.; Panda, D. Genetic improvement of giant freshwater prawn Macrobrachium rosenbergii: A review of global status. Rev. Aquac. 2022, 14, 1285–1299. [Google Scholar] [CrossRef]
- Fu, H.; Jiang, S.; Xiong, Y. Current Status and Prospects of Farming the Giant River Prawn (Macrobrachium rosenbergii) and the Oriental River Prawn (Macrobrachium nipponense) in China. Aquac. Res. 2012, 43, 993–998. [Google Scholar] [CrossRef]
- Chen, X.; Fan, L.; Qiu, L.; Dong, X.; Wang, Q.; Hu, G.; Meng, S.; Li, D.; Chen, J. Metagenomics Analysis Reveals Compositional and Functional Differences in the Gut Microbiota of Red Swamp Crayfish, Procambarus clarkii, Grown on Two Different Culture Environments. Front. Microbiol. 2021, 12, 735190. [Google Scholar] [CrossRef]
- Dong, S.; Gao, Y.; Gao, Y.; He, M.; Liu, F.; Yan, F.; Wang, F. Evaluation of the Trophic Structure and Energy Flow of a Rice-Crayfish Integrated Farming Ecosystem Based on the Ecopath Model. Aquaculture 2021, 539, 736626. [Google Scholar] [CrossRef]
- Nair, C.M.; Salin, K.R.; Joseph, J.; Aneesh, B.; Geethalakshmi, V.; New, M.B. Organic Rice–prawn Farming Yields 20 % Higher Revenues. Agron. Sustain. Dev. 2014, 34, 569–581. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Wang, S.; Ku, X.; Zhong, Z.; Liu, H.; Li, J. Developing Integrated Rice-animal Farming Based on Climate and Farmers Choices. Agric. Syst. 2023, 204, 103554. [Google Scholar] [CrossRef]
- Islam, M.S.; Shofiquzzoha, A.F.M.; Begum, N. Efficacy of Formulated Feed Preference and Stocking Density on Growth and Survival of Baby Pila globosa Reared in Laboratory Condition. Annu. Res. Rev. Biol. 2021, 36, 86–91. [Google Scholar] [CrossRef]
- Moss, A.S.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dawood, M.A.O. Effects of Different Levels of Marine Snail Shells in the Diets of Juvenile Kuruma Shrimps Marsupenaeus japonicus as a Source of Calcium. N. Am. J. Aquac. 2019, 81, 55–66. [Google Scholar] [CrossRef]
- Hossain, M.M.; Chakraborty, S.C. Growth and Economic Analysis of Freshwater Prawn, Macrobrachium rosenbergii (de Man), Produced with Feeds Substituting Sunflower Cake for Fish Meal, Soya Bean Meal and Mustard Oil Cake. Aquac. Res. 2017, 48, 5418–5429. [Google Scholar] [CrossRef]
- Yang, R.; Cao, R.; Gong, X.; Feng, J. Large Shifts of Niche and Range in the Golden Apple Snail (Pomacea canaliculata), an Aquatic Invasive Species. Ecosphere 2023, 14, e4391. [Google Scholar] [CrossRef]
- Roberts, J.K.; Kuris, A.M. Predation and Control of Laboratory Populations of the Snail Biomphalaria glabrata by the Freshwater Prawn Macrobrachium rosenbergii. Ann. Trop. Med. Parasitol. 1990, 84, 401–412. [Google Scholar] [CrossRef]
- Savaya-Alkalay, A.; Ovadia, O.; Barki, A.; Sagi, A. Size-selective Predation by All-male prawns: Implications for Sustainable Biocontrol of Snail Invasions. Biol. Invasions 2018, 20, 137–149. [Google Scholar] [CrossRef]
- Sokolow, S.H.; Lafferty, K.D.; Kuris, A.M. Regulation of Laboratory Populations of Snails (Biomphalaria and Bulinus spp.) by River Prawns, Macrobrachium spp. (Decapoda, Palaemonidae): Implications for Control of Schistosomiasis. Acta Trop. 2014, 132, 64–74. [Google Scholar] [CrossRef]
- Monde, C.; Syampungani, S.; Rico, A.; van den Brink, P.J. The Potential for Using Red Claw Crayfish and Hybrid African Catfish as Biological Control Agents for Schistosoma Host Snails. Afr. J. Aquat. Sci. 2017, 42, 235–243. [Google Scholar] [CrossRef]
- Chimbari, M.J.; Madsen, H.; Ndamba, J. Laboratory Experiments on Snail Predation by Sargochromis codringtoni, a Candidate for Biological Control of the Snails that Transmit Schistosomiasis. Ann. Trop. Med. Parasitol. 1997, 91, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Styles, D.; Cao, Y.; Ye, X. The Sustainability of Rice-crayfish Coculture Systems: A Mini Review of Evidence from Jianghan Plain in China. J. Sci. Food. Agric. 2021, 101, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, H.; Wang, H.; Wu, S.; Bashir, M.A.; Reis, S.; Sun, Q.; Xu, J.; Gu, B. Rice-Animal Co-Culture Systems Benefit Global Sustainable Intensification. Earth’s Future 2023, 11, e2022EF002984. [Google Scholar] [CrossRef]
- Mat Zaib, N.A.N.; Tay, C.; Hashim, S.N.; Mohd Hassan, W.R. Azadirachta indica as Natural Pesticide for Pomacea canaliculata Control: A Review. Sci. Lett. 2023, 17, 1–11. [Google Scholar] [CrossRef]
- Wagiman, F.X.; Ariani Bunga, J.; Hasoloan Purba Sidadolog, J. Sustainable Control of the Golden Snail (Pomacea canaliculata Lamarck) on Irrigated Rice Field in Malaka Regency, East Nusa Tenggara Province, Indonesia. Kne Life Sci. 2019, 4, 156–165. [Google Scholar] [CrossRef]
- Zhang, J.; Quan, G.; Huang, Z.; Luo, S.; Ouyang, Y. Evidence of Duck Activity Induced Anatomical Structure Change and Lodging Resistance of Rice Plant. Agroecol. Sustain. Food Syst. 2013, 37, 975–984. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Dong, L.; Zhang, J. Cleaner Agricultural Production in Drinking-Water Source Areas for the Control of Non-Point Source Pollution in China. J. Environ. Manag. 2021, 285, 112096. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Haїssam Jijakli, M.; Kotzen, B. Towards Commercial Aquaponics: A Review of Systems, Designs, Scales and Nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G. Apple Snails as Animal Feed. In Biology and Management of Invasive Apple Snails, 2nd ed.; Joshi, R.C., Cowie, R.H., Sebastian, L.S., Eds.; Philippine Rice Research Institute (PhilRice): Nueva Ecija, Philippine, 2017; pp. 369–385. [Google Scholar]
- Oficialdegui, F.J.; Sánchez, M.I.; Clavero, M. One Century Away from Home: How the Red Swamp Crayfish Took Over the World. Rev. Fish. Biol. Fish. 2020, 30, 121–135. [Google Scholar] [CrossRef]
- Farmanfarmaian, A.; Moore, R. Diseasonal Thermal Aquaculture— 1. Effect of Temperature and Dissolved Oxygen on Survival and Growth of Macrobrachium rosenbergii. Proc. Annu. Meet.-World Maric. Soc. 1978, 9, 55–66. [Google Scholar] [CrossRef]
- Reid, G.K.; Gurney-Smith, H.J.; Flaherty, M.; Garber, A.F.; Forster, I.; Brewer-Dalton, K.; Knowler, D.; Marcogliese, D.J.; Chopin, T.; Moccia, R.D.; et al. Climate Change and Aquaculture: Considering Adaptation Potential. Aquac. Environ. Interact. 2019, 11, 603–624. [Google Scholar] [CrossRef]
- Savaya-Alkalay, A.; Ndao, P.D.; Jouanard, N.; Diane, N.; Aflalo, E.D.; Barki, A.; Sagi, A. Exploitation of Reproductive Barriers between Macrobrachium Species for Responsible Aquaculture and Biocontrol of Schistosomiasis in West Africa. Aquac. Environ. Interact. 2018, 10, 487–499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).