Abstract
Curculio glandium is one of the pre-dispersal seed predators occurring in Central Europe. It is associated with Quercus robur, the acorns of which are shelter and food sources for developing larvae. Females of the species, to our knowledge, are lacking in marking pheromones or do not use them; therefore, in nature, multiple infestations (over 10 eggs or larvae) of the same host fruit can be found. Such density can provoke very strong competition, which was verified in this study. The survival rate and body mass of 695 second-instar larvae, competing in various test groups (one, three, five, eight and ten larvae) offered one acorn, were measured and video recordings made in order to describe their behavior and determine differences between groups. Experimental observations indicated that when the density of larvae in an acorn increased, the survival rate and body mass significantly decreased—being the lowest in test groups consisting of eight and ten individuals. In the latter groups, also the acorn embryo was completely consumed. Video footage, along with the presence of dead, nibbled larvae and living ones covered with scars resembling mouthparts, is evidence for aggression and cannibalism in the second and the third larval instars—behavior scarce in weevils and in phytophagous insects in general. Results confirm the assumption that in heavily infested oak fruits, competition between individuals is so strong that it involves cannibalism, which at the same time provides the strongest larvae with additional nutrients.
Keywords:
aggression; body mass; diet; density; fitness; Quercus; survival rate; trophic interactions; weevils 1. Introduction
Limitation of resources is one of the crucial factors shaping population growth. In response, organisms perform different strategies aimed at avoiding its negative implications. The superseded theory of group selection [1] assumes that an individual functions only for the benefit of the whole species; therefore, its actions, including competition avoidance, are done solely for this purpose. However, nowadays, and within the context of individual selection [2,3] and the selfish gene theory [4], competition avoidance is explained as a way to increase individual fitness.
Within a competition context, animals should avoid overcrowding with conspecifics or other taxa feeding on the same type of food. However, some examples seem to contradict this principle, such as bark beetles (Curculionidae: Scolytinae) that attract conspecifics by releasing pheromones. The first individuals to infest living trees, the so-called ‘settlers’, intensively inform others about the location of the new resources. Such behavior has been named epideictic (from latin deixis—meaning ‘to mark’). The ‘settlers’ benefit from the presence of the highest possible number of beetles, as their activity causes the tree to die and decay. This provides Scotylinae with an optimal microhabitat for reproduction. The offspring of the ‘settlers’, as the first to hatch, have the greatest chance to complete their development, as they will not suffer resource depletion. On the contrary, the situation is reversed when females encounter already decaying trees—then, no further information is passed, so as to reduce further competition for their offspring [5].
In other insects, epideictic behaviors contribute to shaping the optimal density range, mostly through the release of volatile chemicals, named epideictic pheromones [6]. Epideictic pheromones, recently also named oviposition deterring pheromones (ODP), play a role as guidance for a given female (enabling the even distribution of her own eggs in host tissues) or for other females (to inform her that a given host is already infested) [7]. The first ODP has been described in a parasitoid species, Trichogramma evanescens Westwood, 1833 (Hymentoptera: Trichogrammatidae). Larvae of this wasp develop inside lepidopteran eggs. As, in one ovum, only one individual can develop and larvae are unable to leave the egg and feed somewhere else, Trichogramma females recognize which egg is already occupied.
Avoiding conspecific overcrowding is crucial also for endophytic insects—larvae of which, similarly to parasitoids, are unable to simply leave host plant organs (e.g., seeds, flower buds, leaves) and find another microhabitat to effectively develop in. For instance, larvae of the spotted asparagus beetle Crioceris duodecimpunctata L. (Coleoptera: Chrysomelidae) develop inside an asparagus berry. A single berry can provide food for only one individual, so when a larva encounters an already occupied fruit, it keeps searching for another one [8]. A similar situation can be observed in the European apple sawfly Hoplocampa testudinae Klug, 1816 (Hymenoptera: Tenthredinidae). This species detects volatiles to establish which fruit is already taken by the larva [9]. Other herbivore insects and parasitoids known for utilizing ODPs are, e.g., the alfalfa blotch leafminer Agromyza frontella (Rondani, 1874) (Diptera: Agromyzidae) [10], the root maggot Hylemya spp. (Diptera: Anthomyiidae) [11], the cabbage butterfly Pieris brassicae L. (Lepidoptera: Pieridae) [12], the Mediterranean flour moth Ephestia kuehniella (Lepidoptera: Pyralidae) [13], a wasp Diachasma alloeum (Muesebeck, 1956) (Hymenoptera: Braconidae) [14] and T. ostriniae Pang and Chen, 1974 [15].
Female oviposition behavior and chemical communication can thus reduce competition and improve offspring development conditions. This will in turn increase her own fitness, as, in many insect species, larger larvae are more likely to survive and reproduce [16,17]. Nonetheless, larvae can still act on their own to reduce competition, namely predate con- or heterospecifics feeding on the same discrete food source. Such predation could have a double outcome: on the one hand, it would increase the amount of food, and, on the other, the prey may provide additional nutritional intake. Previous studies have reported on intra- and heterospecific predation among carnivorous parasitoids [18], but also among the larvae of phytophagous insects [19,20].
In Coleoptera, females release ODPs; however, this is not a rule in the case of weevils (Curculionidae), despite the fact that their larvae face, in many cases, intra-specific competition for food [21,22]. The exceptions described so far are the above-mentioned members of the subfamily Scolytinae and the pepper weevil Anthonomus eugenii Cano, 1894 (Curculioninae). Females of the latter species are known for the deposition of a liquid drop after successful oviposition in a flower bud [23]. Competition could nonetheless be reduced by different means, as predation among larvae feeding within the same fruit/seed occurs in different lineages within species-rich Curculionidea weevils [24,25].
We studied intra-specific cannibalism between the larvae of C. glandium Marsham, 1802 (Coleoptera: Curculionidae). This widespread species inhabits oak Quercus spp. forests all over Europe, from the Iberian Peninsula [17] to the north of Europe [26]. In Central Europe, it is the most prevalent Curculio weevil attacking the acorns of the peduncle oak Quercus robur L. (Fagales: Fagaceae) [27]. Curculio glandium females have a specialized long rostrum to drill egg galleries into unripe, green acorns [28,29], or into mature but cracked fruits [30]. Larvae have to develop within a single acorn, feeding on cotyledons until the fourth instar emerges and leaves the fruit to burrow itself into the soil for pupation.
The endoparasitic nature of larvae leads to competition for food with conspecifics developing within the same seed/fruit. However, there are no reports of ODPs released by females either in these insects or other species of the Curculionini tribe. Previous observations on female oviposition behavior show the lack of ODPs in C. glandium. Moreover, under natural conditions, even 11–12 larvae can simultaneously occupy one acorn, supporting the absence of marking pheromones (M.R., personal observation). This is coincident with previous studies on the oviposition behavior of C. elephas, whose larvae develop within chestnuts and acorns. In spite of the negative effects of competition for food on larval size [16,22], females do not release OPDs. Instead, C. elephas usually lay only one egg per seed, but other females may lay within the same seed, increasing the number of larvae developing together [16].
Intra-specific competition could also be regulated by means of larval cannibalism, but we lack information in this regard for C. glandium, and, in C. elephas, it has been disregarded (see [16] for C. elephas). However, at least in one species of the same subfamily (Curculioninae), namely Anchylorhynchus eriospathae Bondar, 1943, cannibalism among early-stage larvae has been demonstrated and observed directly [31]. This is why, although the number of larvae per acorn in natural conditions could suggest that it does not occur in C. glandium, we aimed at studying it directly based on observations and laboratory experiments in which the number of larvae per acorn was manipulated. Acorn multi-infestation would be expected to reduce the larval final size, but also to increase cannibalism among conspecifics if it occurred. We specifically assessed whether (i) mean larval size decreased along with the number of larvae per acorn; (ii) larval survival was lower when more larvae shared the same acorn due to cannibalism; (iii) larval cannibalism—if any—occurred only at certain larval growth stages.
2. Material and Methods
2.1. Effects of the Number of C. glandium Larvae per Acorn on Survival Rate and Body Mass
Research was conducted over two reproductive seasons, from 2013 to 2014. Larvae and acorns were collected from the peduncle oak Q. robur trees growing at the university campus (Warsaw, Poland), starting from mid-August. This particular period guaranteed that only C. glandium larvae were present, instead of C. venosus or C. pellitus. Larvae of the three species are impossible to be distinguished, but C. venosus and C. pellitus reproduce one month earlier so their larval development is completed before the middle of August [32]. Identification by means of DNA barcoding of 20 larvae collected for a different experiment confirmed that only C. glandium was present at these dates in the study area [Reut et al., in prep.].
We followed a manipulative approach to assess the effects of acorn multi-infestation on larval survival and performance. To do so, we placed a different number of larvae in the same development stage within uninfested experimental acorns. Larvae were taken from infested acorns collected in the field. Weevil-infested acorns can be easily recognized based on the oviposition scar remaining after a female’s activity [22,32]. We searched for acorns with these characteristics, but avoided those with markings suggesting that there were larvae of other important acorn predators, namely the caterpillars of Cydia spp. moths (Lepidoptera: Tortricidae). Acorns were precisely opened to obtain the larvae. Usually, one acorn contains larvae at different life stages—from newly hatched to fully mature ones. The second-instar larvae (Figure 1) were chosen for the experiment, mainly because the first one is yet too fragile, whereas the third and the fourth would have finished development too soon.
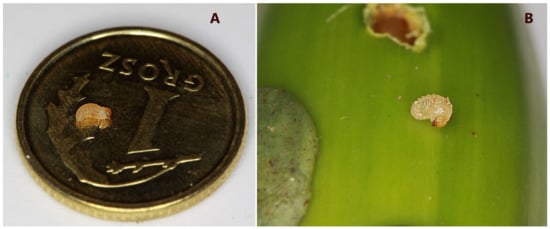
Figure 1.
Larvae of the second instar with light brown head capsule, which indicates that the individual is newly molted: (A)—larva on a coin of 15.5 mm diameter; (B)—larva next to the prepared infestation hole.
Selected larvae were introduced into fresh acorns, the surface of which was carefully checked to make sure that there were no previous scars made by weevils or chestnut tortrix Cydia splendana. All fruits were collected at the same, mature (~80 years old) oak, in order to eliminate the potential bias caused by differentiated levels of tannins depending on the tree. Acorns were also weighted in order to place the different numbers of larvae in fruits as similarl as possible. This was mandatory to assess the effect of the competition on larval performance while controlling for the effect of acorn size [22]. Experimental acorns were pierced with a fine needle that produced a wide hole in which we carefully placed the larva. After this, the openings were plugged with a modeling clay (Figure 2). Five categories were established for the number of larvae—one, three, five, eight, and ten larvae per acorn. We did not introduce more than ten larvae due to technical difficulties in producing so many independent holes in the same experimental acorn.

Figure 2.
Experimental habitat for larvae: (A)—artificially pierced fruit prepared for infestation; (B)—infested acorn with holes closed with clay.
Each infested acorn was assigned a number. The date and number of larvae introduced were noted. Finally, acorns were placed separately in plastic vials filled with a thin layer of moist sand at the bottom. Acorns were checked daily. When developed larvae appeared in the sand, they were weighted to the nearest 0.1 mg and the number of the acorn was recorded. Besides this, four nourishment categories ((I) normal—above 0.039 g, (II) slightly malnourished—0.038–0.029 g, (III) malnourished—0.028–0.021 g, (IV) starved—below 0.020 g) were established on the basis of recorded larval masses for a further analysis of larval mass variability within the different types of experimental acorns. One month after the last larva emerged, acorns were cut open in order to inspect whether any dead individuals remained inside.
2.2. Aggressive Behavior in C. glandium Larvae
Larvae excluded from the density experiment—the first and the third, rarely the second instar (as the majority of them were introduced into acorns)—were placed together on a plastic plate. Such exposed larvae were crawling intensively, encountering each other. Encounters (>50 trays with few larvae) were documented, described and subsequently compared with behavior of the fourth-instar larvae (after the development in the acorn), also placed together with younger ones (>50 trays with few larvae). Video documentation was made with two internet protocol (IP) cameras (Day/Night Megapixel IP Camera, Novus, Poland) connected to a computer and an external disc drive.
2.3. Statistical Analysis
Results were statistically processed using Statistica software (v. 13.0, StatSoft Inc., Tulsa, OK, USA). The normality of the distribution (Shapiro–Wilk’s test, p < 0.05) and the homogeneity of variance (Levene’s test, p < 0.05) were checked. As a result, non-parametric statistics were selected for the analysis. Kruskal–Wallis’ ANOVA with post-hoc Dunn’s test were used in order to determine significant differences (p < 0.05) between survival rates and masses of larvae developing inside acorns with different numbers of larvae. Spearman’s correlation coefficient (rs) was calculated to determine the significance of the relationship (p < 0.05) between the larval density per acorn and their survival rate and body weight. Log-linear tests were used to compare the proportion of experimental acorns in which the cotyledons were depleted depending on the number of larvae. The same test was used to compare the frequencies of larval weight categories among the different types of experimental acorns.
3. Results
3.1. Density of C. glandium Larvae vs. Survival Rate and Body Mass
The number of larvae per acorn was negatively related to their survival likelihood (H(4) = 57.83, p < 0.001) (Table 1). The survival rate decreased significantly when more than five larvae were placed within the same experimental acorn. Post-hoc Dunn’s test showed no significant differences (p > 0.05) in larvae survival rate among one, three and five larvae per acorn, with the highest median survival rate (100%). Thus, most individuals could effectively leave the fruit. A higher density of larvae resulted in significantly increased mortality (post-hoc, p < 0.001). When the number increased to eight and ten larvae per acorn, median survival decreased to 75% and 80%, respectively. In some of these acorns, half of the larvae did not complete their development (Table 1). Additionally, in the fruits containing eight and ten larvae, neither a single piece of acorn embryo nor dead weevil larvae were found.

Table 1.
Larval survival rates at different population densities.
Larval mass also differed among the classes of experimental acorns (Table 2, Figure 3). Kruskal–Wallis’ test showed that the mean larval body mass decreased when the number of larvae per acorn increased (H(4) = 87.62, p < 0.001). No significant differences were found between acorns inhabited by one, three and five individuals (post-hoc, p > 0.05). However, there were significant differences between these and those occupied by eight and ten larvae (post-hoc p < 0.05 in both cases) (Figure 3). Larval weight was the highest in larvae developing singly, without competition (median 0.48 g), whereas individuals from the most crowded acorns (eight and ten larvae per fruit) were lighter by c. 29%. Spearman’s correlation coefficient proved that the larval density in the acorn negatively affected both the larval survival rate (rs = −0.69, p < 0.05) and body mass (rs = −0.83, p < 0.05).

Table 2.
Larval body mass (in grams) in acorns with experimental acorns with different numbers of larvae.
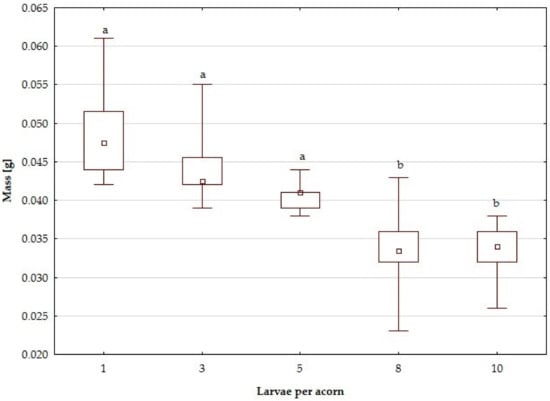
Figure 3.
Distribution of larval masses in acorns inhabited by one, three, five, eight and ten larvae, respectively. Markings: squares—medians, box—quartile range (25–75%), whiskers—min/max values; different lowercase letters indicate significant differences (Dunn’s post-hoc, p < 0.05).
Larval weight decreased because the likelihood of cotyledon depletion increased as the number of larvae per acorn increased (Chi-sq = 126.24, df = 4, p < 0.0001). In the acorns with one and three larvae (N = 20 in both cases), there was always a cotyledon uneaten after larvae finished their development. In acorns with five larvae (N = 15), this happened only in 26% of the cases, whereas in those with eight and ten larvae (N = 30 at each category), the cotyledons were always completely depleted (100%). Accordingly, besides the lower mean larval weight, the comparison of the distribution of larval mass categories among acorns containing three, five, eight and ten larvae differed (Chi-sq = 41.58, df = 9, p < 0.0001). In the last two categories (ten and eight larvae per acorn), there was a small proportion of starved larvae (Figure 4), which did not occur in those in which three and five larvae grew together. In heavily multi-infested acorns (eight and ten larvae), there were also more larvae classified as malnourished (22% vs. 17%, respectively). In these acorns, the percentage of larvae that achieved a normal size was only slightly over 25%; by contrast, in acorns with three and five, more than half of the larvae were in this category (over 70% in the case of three larvae acorns) (Figure 4).
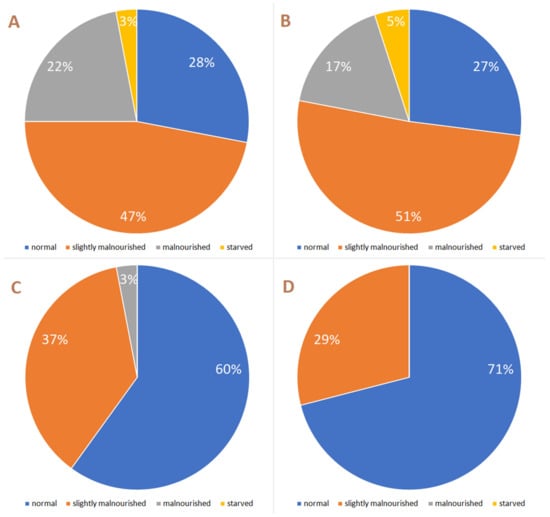
Figure 4.
Larval mass categories in experimental acorns with a different numbers of larvae: (A)—ten larvae; (B)—eight larvae; (C)—five larvae; (D)—three larvae. The percentages are based on a sample size of 58, 67, 186 and 22 larvae, respectively.
3.2. Aggressive Behavior in C. glandium Larvae
Immature larvae (i.e., the third and the second instars) tended to be very aggressive towards one another while crawling together on a plate. They commonly used their mouthparts to bite conspecifics. Many times, this resulted in the death of the attacked one. Larvae of the fourth instar, in turn, acted completely differently. Individuals placed together on a plate showed no aggression. They did not use their mouthparts to stab others but tried to simply move away.
In Figure 5, the phases of a typical attack are illustrated: (A)—the third-instar larva encounters the second instar; (B)—older larva opens its mouthparts and tries to bite; if it succeeds, the aggressor bends its body to move closer to its opponent; (C)—in this position, the larger individual bites many times the lower parts of the smaller one. After a while, hemolymph oozes from the body of the bitten larva. A complete attack is visible in the attached video. In addition, we noticed that some of the larvae that managed to survive were covered in scars, the shape of which resembled the mouthparts (Figure 5E,F). Post-experimental opening of the acorns revealed dead, partially eaten individuals inside fruits infested by three and five larvae (Figure 5D).
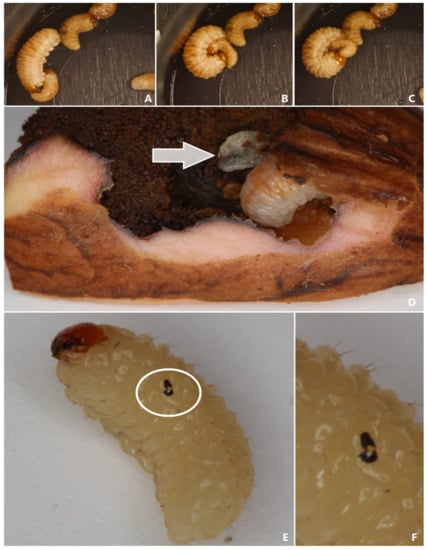
Figure 5.
Aggressive behavior of C. glandium larvae: (A–C)—subsequent stages of an attack; (D)—dead, partly eaten larva (marked with an arrow) and well-fed, living one; (E)—scar on the body of a fully developed larva; (F)—close-up of the mouthpart-shaped scar.
3.3. Consequences of the Reduction of the Number of Larvae by Conspecific Attack on C. glandium Growth and Development
In some experimental acorns with an initial number of larvae of five, eight and ten, the final number of larvae was lower than the initial one, presumably due to conspecific attack. In the case of those with an initial number of larvae of five, the reduction in the number of larvae benefited the remaining ones (H(4) = 6.35, p = 0.04) (medians 0.044, 0.041 and 0.040 g for acorns with three, four and five larvae at the end). This did not happen in the case of acorns with an initial number of eight and ten larvae. In addition, the initial number of larvae affected their final mass, even if the final number of larvae was the same. In acorns in which five larvae emerged but there were also five larvae at the beginning, the mean weight was lower in those that had more larvae initially (H(2) = 12.15, p < 0.01) (Figure 6). When the number of larvae was over five, the initial number of larvae had no significant effect in either case.
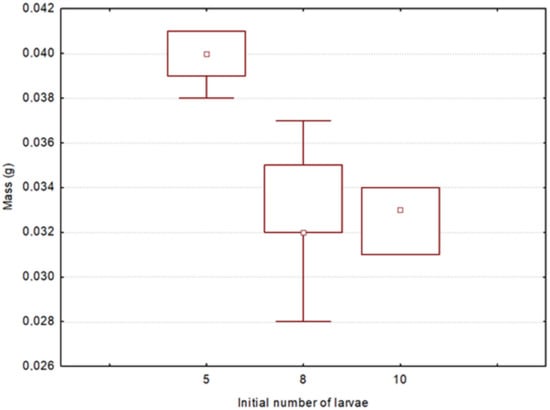
Figure 6.
Distribution of larval masses in acorns with a final number of five larvae per acorn and an initial one of five, eight and ten larvae. Markings: squares—medians, box—quartile range (25–75%), whiskers—min/max values.
4. Discussion
The negative relationship between larval weight and the number of larvae per acorn confirms that food availability constrains larval growth (see [16,22] for similar results for C. elephas). Larval size at development completion is determined by both the number of larvae per fruit and fruit size [22]. As experimental acorns had a similar size, larval weight decreased significantly when more than five larvae grew together. Larval size is a good proxy for future individual fitness in insects in general [33] and also in acorn Curculio [16,34]. Hence, as larvae have to complete their development within the acorn into which the egg is laid, female oviposition behavior should optimize the resources available for larval growth. This can be achieved by choosing large fruits for oviposition and/or reducing the number of eggs per fruit.
Females of some Curculionidae, such as the rice weevil Sitophilus oryzae (L.) (Curculionidae), have been shown to choose larger grains for egg deposition [35]. In Curculio weevils, C. elephas does not choose larger ones when it oviposits into Castanea sativa chestnuts [36]; however, it does in the holm oak Q. ilex acorns [R. Bonal, in prep.], which are, on average, smaller than chestnuts. Information on fruit size selection in C. glandium is still lacking, but our results confirm that multi-infestation still has costs. Individual females could try to avoid this by reducing the clutch size per fruit and, at least, reducing competition among siblings. This happens in C. elephas, in which females usually lay one egg per fruit [16]; by contrast, C. glandium females in nature usually lay more eggs than only one per acorn [37]. However, the present study shows that, although C. glandium larvae need less food per capita than the larger C. elephas ones [17], the clutch size per fruit should be limited in any acorn weevil species.
Competition among siblings can be reduced by laying fewer eggs per fruit, but larvae may still have to compete if they have to grow together with non-related conspecifics. This happens, for example, in the walnut husk fly Rhagoletis juglandis Cresson, 1920 (Diptera: Tephritidae) associated with the walnut Juglans major (Torr.) A. Heller (Fagales: Juglandaceae). Females add their own eggs into nuts already occupied by other females, despite the presence of oviposition-deterrent pheromones (ODP). This results in a reduced survival rate and lower body mass at the pupation stage of larvae developing in the most crowded fruits [38]. In C. glandium, larvae developing inside one acorn are often not related, inasmuch as females can lay eggs into already taken fruits [M. Reut, pers. obs.], as in the congeneric C. elephas, which does not deposit any ODP [16]. As time passes, the acorn resources are dwindling and, as a consequence, less ovipositional sites are available for acorn weevils [21]. Under such conditions, they risk overcrowding for their offspring by laying eggs into available fruits. Therefore, it is very likely that overpopulated acorns are a site of severe competition within unrelated offspring. Especially since, as previously stated, in the beginning, up to 12 larvae can forage in one oak fruit.
Aggression and cannibalism were directly observed among larvae taken outside the acorn. To the best of our knowledge, this is the first time that this behavior has been recorded in Curculio weevils, and it was concentrated in larvae of the second and the third instars, whereas it did not happen in the first and disappeared in the fourth (and the last) instars. This instar-dependent behavior resembles that of palm-associated curculionids—A. eriospathae and Revena rubiginosa Boheman, 1936. In A. eriospathae, for instance, it is the first-instar larva that typically kills all the competitors (and unhatched eggs) within one seed. In this species, the aggressive larvae had, at this instar, morphological adaptations for killing other larvae, specifically falcate mandibles. Later, as the endosperm becomes harder, in further molts, mandibles are reshaped into a scraping tool, enabling food intake [31]. In the case of C. glandium, such a dramatic morphological change does not occur, and larvae of all instars can feed on mature cotyledons [30,33], so no morphological adaptations are required. Therefore, in our view, cannibalism in C. glandium can occur but is not the rule, as in A. eriospathae, and, as our results show, it depends on the number of larvae per acorn.
The absence of oviposition-deterrent pheromones means that C. glandium females cannot always prevent further competition among larvae; however, larvae could control it to some extent by killing conspecifics within the same fruit. Our results show that development failure (presumably due to conspecific aggression) is extremely rare in acorns with five larvae or less. However, it quickly increases and, in acorns with ten larvae, there was not a single one in which all the larvae completed their development successfully. The reduction in the number of larvae did not prevent cotyledon depletion, however, which means that the potential of conspecific predation to reduce competition is limited. For example, in acorns with an initial number of five larvae, cotyledons were fully consumed only in 26% of the cases, whereas in those with eight larvae, it always occurred, even in those in which the final number of larvae was five. Accordingly, the mean larval weight was also significantly lower than in those that had five larvae from the beginning. The reduction in the number of larvae only had a positive effect on the mean larval weight in acorns that initially had five and finally three, because the cotyledons were not depleted. The reason that the reduction in the number of larvae does not prevent cotyledon depletion in overcrowded acorns is because, when the larvae are killed (second and third instars), they have already reached a medium size and have eaten a significant amount of cotyledons that cannot be used by conspecifics.
Conspecific predation could have an additional benefit if it provides a food source to compensate for cotyledon depletion. On average, the data show that in overcrowded acorns, cannibalism does not increase the mean larval weight. However, the consumption of dead larvae might explain why, even in acorns that initially contained ten larvae, some of them completed their development well fed, while others were starving. Such variability was not recorded in acorns with three and five larvae and might indicate that well-fed ones in large acorns might have predated conspecifics. Cannibalism might supplement the strongest individuals with additional nutrients, some of which (e.g., proteins) could be very important. Benefits of protein enrichment in the diet of herbivore insects have been considered as adaptive—for example, in the case of the maize weevil Sitophilus spp., in which cannibalism has been reported [39]. A diet enriched with proteins accelerated the development, and adult individuals that emerged from the cannibal larvae were found to be heavier and subsequently produced more and better-quality progeny [25,39]. Cannibalism might compensate for the effects of some secondary plant compounds, such as tannins, whose effects on protein digestion have been studied intensively, both on vertebrates [40,41] and insects [42,43]. Nonetheless, modern views on this matter suggest no negative effect on insect feeding, contrary to vertebrates [44].
We acknowledge that direct aggression between C. glandium larvae was observed when they were taken out of the acorns, but our data suggest that the same occurred inside. Aggression was additionally evidenced by scars resembling the shape of mouthparts on the bodies of the surviving larvae (Figure 5E,F). Finally, having dissected the experimental acorns, we found only partially eaten individuals in the few acorns with three and five larvae placed at the beginning (Figure 5D). By contrast, in the experimental groups consisting of eight and ten individuals, no fragments of bodies were observed, suggesting that all available food was completely consumed. It is true that survivors could have consumed the bodies of those that had died from starvation, but both our behavioral observations and reports from other Curculionids suggest that it occurs after a conspecific attack. In the case of the maize weevil Sitophilus zeamais (Motschulsky, 1855) and the grain weevil S. granarius (L.), this has been recorded even when they are inside the acorns by means of X-ray scanning photography. Moreover, in agreement with our results, in both species, cannibalism took place when the larvae faced limited resources.
5. Conclusions
Our data show, for the first time, conspecific aggression and cannibalism in the acorn Curculio weevils. It occurred only among larvae at the second and third instars, and mostly in experimental acorns in which more than five larvae were placed to grow together. Cannibalism may reduce competition for food but only to some extent, as, in overcrowded acorns, the size of surviving larvae was, on average, lower despite conspecific aggression. This occurred because larvae were predated when they had reached a medium size and cotyledons were depleted despite the reduction in the number of larvae per acorn. However, predated conspecifics might still provide a small nutritive supplement to the strongest larvae, which were well fed at the end, in contrast to the extraordinarily small, starving larvae, also found in these overcrowded acorns. This study provides a key starting point for further work exploring the behavioral ecology of this and other acorn Curculio spp., the most prevalent pre-dispersal acorn predators in oak forests worldwide.
Author Contributions
Conceptualization, M.R.; methodology, M.R.; software, M.R. and M.C.; formal analysis, M.C.; investigation, M.R. and H.M.; resources, M.R.; data curation, M.R., H.M. and M.C.; writing—original draft preparation, M.R., H.M. and R.B.; writing—review and editing, H.M.; visualization, M.R. and M.C.; supervision, M.R.; project administration, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wynne-Edwards, V.C. Animal Dispersion in Relation to Social Behavior; Oliver & Boyd: London, UK, 1962. [Google Scholar]
- Smith, J.M. Group selection and kin selection. Nature 1964, 201, 1145–1147. [Google Scholar] [CrossRef]
- Grafen, A. Natural selection, kin selection and group selection. In Behavioural Ecology: An Evolutionary Approach; Krebs, J., Davies, N., Eds.; Blackwell Scientific Publications: Oxford, UK, 1984; pp. 62–84. [Google Scholar]
- Dawkins, R. The Selfish Gene; Oxford University Press: New York, NY, USA, 1976. [Google Scholar]
- Alcock, J. Natural selection and communication among bark beetles. Fla. Entomol. 1982, 65, 17–32. [Google Scholar] [CrossRef]
- Prokopy, R.J. Epideictic pheromones that influence spacing patterns of phytophagous insects. In Semiochemicals: Their Role in Pest Control; Wiley: New York, NY, USA, 1981; pp. 181–213. [Google Scholar]
- Hoffmeister, T.S.; Roitberg, B.D. Evolutionary ecology of oviposition marking pheromones. In Chemoecology of Insect Eggs and Egg Deposition; Blackwell: Berlin, Germany, 2002; pp. 319–347. [Google Scholar]
- Van Alphen, J.J.M.; Boer, H. Avoidance of scramble competition between larvae of the spotted asparagus beetle, Crioceris duodecimpunctata L. (Chrysomelidae) by discrimination between unoccupied and occupied asparagus berries. Neth. J. Zool. 1979, 30, 136–143. [Google Scholar]
- Roitberg, B.; Mangel, M.; Lalonde, R.; Roitberg, C.; van Alphen, J.; Vet, L. Seasonal dynamic shifts in patch exploitation by parasitic wasps. Behav. Ecol. 1992, 3, 156–165. [Google Scholar] [CrossRef]
- Mcneil, J.N.; Quiring, D.T. Evidence of an oviposition-deterring pheromone in the alfalfa blotch leafminer, Agromyza frontella (Rondani) (Diptera: Agromyzidae). Environ. Entomol. 1983, 12, 990–992. [Google Scholar] [CrossRef]
- Zimmerman, M. Oviposition behavior and the existence of an oviposition-deterring pheromone in Hylemya. Environ. Entomol. 1979, 8, 277–279. [Google Scholar] [CrossRef]
- Rothschild, M.; Schoonhoven, L.M. Assessment of egg load by Pieris brassicae (Lepidoptera: Pieridae). Nature 1977, 266, 352–355. [Google Scholar] [CrossRef]
- Corbet, S.A. Oviposition pheromone in larval mandibular glands of Ephestia kuehniella. Nature 1973, 243, 537–538. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Oakleaf, R.; Rodriguez-Saona, C. Oviposition-deterring pheromone deposited on blueberry fruit by the parasitic wasp, Diachasma alloeum. Behaviour 2007, 144, 429–445. [Google Scholar]
- Yong, T.H.; Pitcher, S.; Gardner, J.; Hoffmann, M.P. Odor specificity testing in the assessment of efficacy and non-target risk for Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Biocontrol. Sci. Technol. 2007, 17, 135–153. [Google Scholar] [CrossRef]
- Desouhant, E.; Debouzie, D.; Ploye, H.; Menu, F. Clutch size manipulations in the chestnut weevil, Curculio elephas: Fitness of oviposition strategies. Oecologia 2000, 122, 493–499. [Google Scholar] [CrossRef]
- Bonal, R.; Espelta, J.M.; Vogler, A.P. Complex selection on life-history traits and the maintenance of variation in exaggerated rostrum length in acorn weevils. Oecologia 2011, 167, 1053–1061. [Google Scholar] [CrossRef]
- Harvey, J.A.; Poelman, E.H.; Tanaka, T. Intrinsic inter-and intraspecific competition in parasitoid wasps. Annu. Rev. Entomol. 2013, 58, 333–351. [Google Scholar] [CrossRef]
- Denno, R.F.; McClure, M.S.; Ott, J.R. Interspecific interactions in phytophagous insects: Competition reexamined and resurrected. Annu. Rev. Entomol. 1995, 40, 297–331. [Google Scholar] [CrossRef]
- Vanitha, S.; Kumar, P.; Jayanthi, P.D. Existence of cannibalism in south american leaf miner, Tuta absoluta (Meyrick). Pest Manag. Hortic. Ecosyst. 2020, 26, 272–273. [Google Scholar]
- Bonal, R.; Muñoz, A. Negative consequences of premature seed abscission on insect performance: Acorn growth suppression constrains Curculio elephas larval size. Ecol. Entomol. 2008, 33, 31–36. [Google Scholar]
- Bonal, R.; Munoz, A. Seed weevils living on the edge: Pressures and conflicts over body size in the endoparasitic Curculio larvae. Ecol. Entomol. 2009, 34, 304–309. [Google Scholar] [CrossRef]
- Addesso, K.M.; McAuslane, H.J.; Stansly, P.A.; Schuster, D.J. Host-marking by female pepper weevils, Anthonomus eugenii. Entomol. Exp. Appl. 2007, 125, 269–276. [Google Scholar] [CrossRef]
- Oberprieler, R.G.; Marvaldi, A.E.; Anderson, R.S. Weevils, weevils, weevils everywhere. Zootaxa 2007, 1668, 491–520. [Google Scholar] [CrossRef]
- Guedes, N.M.P.; Guedes, R.N.C.; Campbell, J.F.; Throne, J.E. Contest behaviour of maize weevil larvae when competing within seeds. Anim. Behav. 2010, 79, 281–289. [Google Scholar] [CrossRef]
- Balalaikins, M.; Telnov, D. Latvian Curculioninae (Coleoptera: Curculionidae): 3. Tribe Curculionini Latreille, 1802. Zool. Ecol. 2012, 22, 181–189. [Google Scholar] [CrossRef]
- Wanat, M.; Mokrzycki, T. The Checklist of the Weevils (Coleoptera: Curculionoidea) of Poland Revisited. Ann. Zool. 2018, 68, 1–48. [Google Scholar] [CrossRef]
- Forrester, G.J. The Population Ecology of Acorn Weevils and Their Influence on Natural Regeneration of Oak. Doctoral Dissertation, University of London, London, UK, 1990. [Google Scholar]
- Rohlfs, D.A. A Study of Acorn Feeding Insects: Filbert Weevil (Curculio occidentis (Casey)) and Filbertworm (Cydia latiferreana (Walsingham)) on Garry Oak (Quercus garryana) (Dougl.) in the Southeastern Vancouver Island Area. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 1999. [Google Scholar]
- Reut, M.; Moniuszko, H. Atypical Oviposition in Curculio glandium Marsham (Coleoptera: Curculionidae)—A Response to Unfavorable Environmental Conditions? Col. Bull. 2022, 76, 36–38. [Google Scholar] [CrossRef]
- De Medeiros, B.A.S.; de Cássia Bená, D.; Vanin, S.A. Curculio Curculis lupus: Biology, behavior and morphology of immatures of the cannibal weevil Anchylorhynchus eriospathae GG Bondar, 1943. PeerJ 2014, 2, e502. [Google Scholar] [CrossRef] [PubMed]
- Reut, M.; Chrabąszcz, M.; Moniuszko, H. Timing Is Everything. Temporal and Spatial Niche Segregation in Curculio spp. (Coleoptera: Curculionidae) Associated with Oak Trees. Insects 2021, 12, 687. [Google Scholar] [CrossRef]
- Songvorawit, N.; Butcher, B.A.; Chaisuekul, C. Feeding performance of the larval stag beetle Aegus chelifer (Coleoptera: Lucanidae) explains adult body size variation and sexual dimorphism. Can. Entomol. 2022, 154, e2. [Google Scholar] [CrossRef]
- Bonal, R.; Hernandez, M.; Ortego, J.; Munoz, A.; Espelta, J.M. Positive cascade effects of forest fragmentation on acorn weevils mediated by seed size enlargement. Insect Conserv. Divers. 2012, 5, 381–388. [Google Scholar] [CrossRef]
- Campbell, J.F. Influence of seed size on exploitation by the rice weevil, Sitophilus oryzae. J. Insect Behav. 2002, 15, 429–445. [Google Scholar] [CrossRef]
- Desouhant, E. Selection of fruits for oviposition by the chestnut weevil, Curculio elephas. Entomol. Exp. Appl. 1998, 86, 71–78. [Google Scholar] [CrossRef]
- Reut, M.; Jakubczyk, E.; Chrabąszcz, M.; Moniuszko, H. Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae). Diversity 2022, 14, 922. [Google Scholar] [CrossRef]
- Nufio, C.R.; Papaj, D.R. Superparasitism of larval hosts by the walnut fly, Rhagoletis juglandis, and its implications for female and offspring performance. Oecologia 2004, 141, 460–467. [Google Scholar] [CrossRef]
- Bolívar-Silva, D.A.; Guedes, N.M.P.; Guedes, R.N.C. Larval cannibalism and fitness in the stored grain weevils Sitophilus granarius and Sitophilus zeamais. J. Pest Sci. 2018, 91, 707–716. [Google Scholar] [CrossRef]
- McArt, S.H.; Spalinger, D.E.; Collins, W.B.; Schoen, E.R.; Stevenson, T.; Bucho, M. Summer dietary nitrogen availability as a potential bottom-up constraint on moose in south-central Alaska. Ecology 2009, 90, 1400–1411. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S. Comparative aspects of plant tannins on digestive physiology, nutrition and microbial community changes in sheep and goats: A review. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1181–1193. [Google Scholar] [CrossRef]
- Felton, G.W. Nutritive quality of plant protein: Sources of variation and insect herbivore responses. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 1996, 32, 107–130. [Google Scholar] [CrossRef]
- Barbehenn, R.; Cheek, S.; Gasperut, A.; Lister, E.; Maben, R. Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midgut fluids of Malacosoma disstria and Orgyia leucostigma caterpillars. J. Chem. Ecol. 2005, 31, 969–988. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).