Abstract
About 20 species of non-native mammals have been recorded in Poland. Some of them are already extinct or have been extirpated, while others are widely distributed and may affect the native biota in Poland. We review the literature on 15 non-native species found in this country, discussing their origin, distribution, and presence on lists of invasive species that pose a threat to wildlife in Poland and the EU. In addition, we discuss current knowledge on their impact on Polish ecosystems. However, on many of these species, there is little information, and the consequences of their presence remain unclear. Therefore, we emphasize the importance of this review for appropriate species management and suggest the introduction of monitoring, especially of species whose populations are increasing.
Keywords:
biodiversity; competition; distribution; impact; introduction; invasive species; mammals; non-native species 1. Introduction
Anthropogenic activities play an important role in shaping ecosystems, as they lead to changes in the distribution of many species worldwide. While some of these changes are minor, others, such as the introduction of non-native species, can have a significant impact not only on the biota of a region but also on the people living there. Accurate predictions of the long-term impact of non-native species on ecosystems and biodiversity are difficult to make, further complicating management decision making.
The arrival of an alien species can have a significant impact on the native biota in that, among other things, it constitutes additional competition for native species, may hybridize with them, and may introduce non-native parasites. In some cases, when natural conditions favor an alien species, e.g., through the absence of a predator preying on the species in its native range or the absence of parasites limiting its distribution, biological invasion may occur, which can have a detrimental effect on native biodiversity [1]. Moreover, invasions by alien species are steadily increasing as a result of their accidental movement by human agencies, intentional introduction, or the international animal trade [2].
Despite the global scale of this phenomenon, involving a large number of animal species over a vast geographic area, the processes underlying the success of invasive species are still poorly understood and require detailed research. Two critical stages in the invasion of a non-native species are its introduction and its spread in a new region. Introduced individuals may come from several genetically distinct sources, and such an admixture of individuals with distinct origins can enhance their genetic and adaptive variability and viability [3,4,5]. On the other hand, in the absence of adaptive challenges, some species with low genetic diversity can spread easily [6]. Nevertheless, in order to limit the spread of non-native species, we need information on the biology, pathways of introduction, sources of origin, and genetic structure of invasive species in order to implement effective methods of preventing such invasions or at least managing them [7,8].
The growing problem of invasive alien species has received attention in the European Union. The List of Invasive Alien Species of Union Concern is an important part of EU Regulation 1143/2014 on Invasive Alien Species, which provides guidelines for actions and decisions regarding IAS in the member states of the EU [9]. Three types of measures are described: prevention, early detection, and rapid eradication and management. For each of the listed species, a short description with relevant information is provided, as is specific information on the measures that should be taken for the species.
The main purpose of this article is to review the literature concerning Polish research on invasive alien species, in order to update knowledge of mammal species considered to be non-native in Poland.
2. Methodology
In this paper, we discuss 15 species of mammals considered to be non-native in Poland (Table 1). Our inclusion of these species is based on two criteria. First, we describe alien species that are currently occurring in Poland or were documented no earlier than the 20th century but are now extinct. Second, we include non-native species that have long been part of the Polish fauna, such as the house mouse and house rat, but are considered alien or invasive by the scientific community, e.g., the Institute of Nature Conservation of the Polish Academy of Sciences, Kraków [10]. We exclude species whose appearance was the result of individual escapes from breeding, e.g., degu (Octodon degus). The species described are considered alien according to the definitions provided by the Convention on Biological Diversity Conference of the Parties (CBD COP 6 Decision VI/23), which defines an alien species as “a species, subspecies, or lower taxon, introduced outside its natural past or present distribution”, and introduction as “the movement by human agency, indirect or direct, of an alien species outside of its natural range (past or present)” [11]. The status of the species includes their ranges from extinct or sporadic occurrence to ubiquitous or widely distributed, with some considered invasive and a potential threat to the biodiversity of the European Union or Poland (Table 1).

Table 1.
Non-native mammals that have been recorded under natural conditions in Poland, along with their current distribution and presence (+) or lack (-) on the List of Invasive Alien Species of Union concern and the List of Invasive Alien Species of Poland concern. Common names are according to the IUCN Red List and ITIS [9,12].
We searched four databases for the relevant literature in October and November 2022: Web of Science, PubMed, Google Scholar, and Google [13,14,15,16]. Instead of applying filters, we entered species names in Polish and English along with scientific names as keywords. Additional keywords specifying the subject matter of the articles were “non-native”, “alien”, “invasive”, “invasion”, “impact”, “ecosystem”, “influence”, “Poland”, and “Polska”. From the results provided by the search engines, we selected those that corresponded with the subject of our article on the basis of the abstract, and then analyzed their references to find related literature that could provide further information relevant to this review. We included 181 sources in our review, 147 of which were Polish or with Polish contributions.
The most abundant Polish literature was found for the raccoon dog (33), raccoon (22), American mink (20), mouflon (15), and fallow deer (12). Overall, the fewest Polish sources were found for rodents and species with sporadic records or for extinct species, e.g., American beaver and Siberian roe deer. Wherever possible, we provide for each species information on (i) its first documented appearance in Poland, (ii) its origin, (iii) its current status (Table 1) and distribution with reference to distribution maps, (iv) its estimated population size, and (v) its documented impact on native biota. On many species, however, little or no research has been carried out. We, therefore, give more attention to some species than others, mainly because of the extensive literature pertaining to the former. For well-studied species, we provide some additional information.
3. Carnivora
3.1. American Mink
The American mink is a semi-aquatic species, endemic to North America, which was introduced into the wild in Europe in the 1930s. Currently, the species is present throughout Poland. The highest population densities have been recorded in the northeast and in the west (Lubuskie and Wielkopolska Provinces), which may be related to the invasion routes of this species into Poland. The species has not been found in the Carpathian Mountains, while the fewest localities with records are in the southeastern areas of the country, i.e., in Podkarpackie, Lublin, Małopolskie, and Świętokrzyskie Provinces [17]. Since 2001, the American mink has been treated as a game animal and its harvesting is monitored (Figure 1). In recent years, there has been a slight decrease in the percentage of districts where American mink have been hunted. Initially, about 2000 animals were harvested, with a peak in the 2015–2016 season. In the 2020–2021 season, the number dropped significantly, falling back to the initial level (Figure 1).
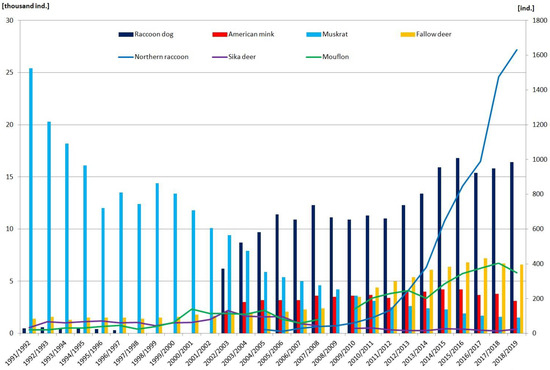
Figure 1.
Hunting harvests of seven species in 1991–2019. The bars show the harvest in thousands of individuals (thousand ind.), while the solid lines show the exact number of individuals harvested (ind.). The gaps in data continuity for mouflon and sika deer are because they were not hunted in those years, while the lack of data for the raccoon dog is because it was not listed as a game species [18,19].
There are many sources of origin of the wild American mink population in Poland, but they were mainly animals that migrated from the population released in Belarus and from the Lithuanian population. They colonized northeastern Poland [20,21]. On the other hand, the source of the population in the northwestern areas of the country may have been mink that escaped from some of the many fur farms in the area.
The rate of expansion of these animals is variable and the population in a given area reaches its maximum density after about 10–15 years. The populations analyzed in Poland exhibit high genetic variability and variation between subpopulations in different regions of the country. This is atypical for alien and invasive animals; because the founder group was small, the variability should be low [20,21,22,23,24]. Studies indicate that the various populations of the American mink differ significantly, in both population density and morphometric traits such as weight or body length. It has also been shown that mink quickly recolonize sites from which they were previously eliminated [25].
The diet of the American mink in Poland varies greatly and is highly dependent on the type of habitat and the area of occurrence. In Lower Silesia (the Barycz Valley), a high proportion of rodents, mainly Microtus spp., was found (in 88.3% of the total feces analyzed). Mink also readily fed on fish, birds, and amphibians, whereas insects, crayfish, and reptiles made up only a small part of the biomass. There were also seasonal differences; in spring and autumn, mink fed mainly on rodents and fish, whereas the winter and summer diet comprised a wide variety of prey [26]. American mink can be a source of parasites, such as those of the genus Trichinella (3.3% among the individuals examined) [27,28].
The appearance of a new predatory species can significantly affect native animal species. A study of the populations of two bird species—coots (Fulica atra) and great crested grebes (Podiceps cristatus)—in the Masurian Lake District in northeastern Poland during the first years following the American mink’s appearance in the area revealed that it had had a significant impact on their distribution and abundance, particularly coots. Since mink avoided habitats near human settlements, these birds achieved better reproductive success in built-up areas along the shores of lakes, as well as when they reproduced in colonies [29]. Alien species can also affect populations of native carnivores through competition. A study of the populations of the American mink and European polecat (Mustela putorius) in northeastern Poland in 1995–2000 showed that the polecat population was more stable during that period, whereas mink numbers declined [30]. Significant differences in habitat use between these species were noted, as well as a different pattern of diurnal activity, which probably translated into the possibility that they could coexist in this small area at relatively high densities and could exploit the same habitats [30]. Mink were much more frequently trapped than polecats. From the data obtained, it was not possible to conclusively demonstrate the effect of the mink’s invasion on the population size of the polecat or on the interaction between the two species [31]. An analysis of the diets of both species conducted in the Bialowieża Primeval Forest showed that both are dominated by amphibians, and that the two predator species exploiting abundant prey can coexist in spite of the very large overlap in their food niches [32].
Population studies of the muskrat (Ondatra zibethicus) carried out in two periods (1996–1998 and 2007) showed that the population size of this species decreased by 44% in the first period and by up to 7% in the second. This was most likely related to predation by the American mink, which is a natural enemy of the muskrat and specializes in hunting it [33]. A similar study of the native European water vole (Arvicola amphibius) in the Masurian Lake District (northeastern Poland) showed that the species was significantly less likely to occur in localities where mink were present [34]. This study indicated that, in postglacial landscapes, the American mink avoids urbanized and mid-field areas away from lakes; thus, such areas may act as refuge habitats for the European water vole and help maintain populations of this rodent despite the mink’s presence [34]
In studies of wildlife damage, mink were present on 52% of fish farms and were widely distributed in eastern Poland. Most surveys showed that mink were resident in ponds for less than 15 years; only one case from northeastern Poland confirmed that mink had been present in a pond since the mid-1970s. Ten respondents representing fish farms were of the opinion that, after the expansion phase, the number of mink in their ponds had decreased significantly over 2–3 years [35]. Similar results were obtained in a study by Manikowska-Slepowronskaya et al. [36], in which an analysis of 104 fish farms showed mink to be present in 31% of them, mainly in ponds fed by rivers.
3.2. Raccoon Dog
The raccoon dog’s original distribution was in Asia, mainly in China, Russia, the Korean Peninsula, Japan, and Mongolia [37]. The species was introduced to the former Soviet Union in the early 20th century. Initially bred for fur, raccoon dogs escaped from farms and spread to continental Europe. Raccoon dogs are classified as an invasive non-native species and a major threat to biodiversity.
Studies of the raccoon dog carried out in Poland to date have examined not only wild populations in order to investigate their size and density, migration routes, ecological parameters, interactions with other predators, and genetic structure [38,39,40,41,42,43,44,45,46,47], but also animals kept on fur farms [47,48,49,50,51,52,53]. Although most of the research on the wild populations took place more than a decade ago, it has been possible to collect basic information on their expansion and their impact on native fauna and its diversity.
In Poland, the raccoon dog is the most widespread alien invasive predator species [44,46]. It started to colonize the country in the first half of the 1950s from an easterly direction (from the former Soviet Union) and gradually expanded westward [54,55]. The hypothesis regarding the expansion of raccoon dogs from the east is supported by the results of phylogenetic studies by Horecka et al. [47]. indicating the existence of two main clades related to continental and island populations; Polish raccoon dogs belong to the former. The relationships among haplotypes within this clade indicate that Polish raccoon dogs are genetically very similar to the Russian population. A similar conclusion was reached by Kasperek et al. [51], who studied genetic diversity in the Polish raccoon dog population using microsatellite sequences.
The first individuals were sighted in the Białowieża Primeval Forest and near Hrubieszów in the Zamość region [56]. At the beginning of the 1960s, single individuals were recorded throughout the country [57]. Over the past 70 years, they have colonized almost the entire area of the country, except for the mountainous areas of southern Poland, such as the eastern part of the Beskid Żywiecki Mts., the Tatra Mts., and the Gorce Mts., as well as the Beskid Sądecki, Beskid Niski, and Bieszczady Mtns. [46,58]. According to Osten-Sacken et al. [59], who studied these animals in western and southwestern Poland, raccoon dogs prefer forest habitats, but they have also been sighted in fields, on river banks and the shores of other water bodies, in wetlands and swamps, along roads, and in human settlements.
The raccoon dog’s territorial expansion went hand in hand with an increase in its population size in Poland—estimated at about 54,500 individuals in 2011 [45,60]. However, most reports on the occurrence and abundance of raccoon dogs come from regional surveys; hence, estimated population sizes of this predator in Poland are difficult to come by. Święcicka et al. [61] stated that, between 2001 and 2008, the raccoon dog population in the Bydgoszcz region (northern Poland) increased by an average of 146 individuals per year. The total population size was estimated at about 2310 individuals, which, in terms of the size of the game predator population in the region, ranked it second after the red fox. According to the Polish Hunting Association, raccoon dogs have been found on 88.8% of the area of Poland, and their density is estimated at 1–5 individuals per km2, depending on the environment [62]. More than two decades ago, Goszczyński [41] reported the density of raccoon dogs in the forests of the Suwałki Landscape Park (north-eastern Poland) to be 0.37 individuals per km2, while, in the Białowieża Primeval Forest (northeastern Poland), the density was 0.5–0.7 individuals per km2 [63]. This indicates a significant increase in the number of individuals in the last 20 years (Figure 1). As the raccoon dog tends to migrate over long distances, it can easily spread to new areas. In addition, it is highly adaptable, has a high reproductive potential, and seems to have found a vacant niche in Poland (and more widely in Europe). Moreover, it has probably benefitted from baited rabies vaccinations, and it is profiting from global warming, as its range limits are governed by extremely low winter temperatures.
Being an invasive species, the raccoon dog can have a negative impact on native fauna and its diversity through competition and predation, as well as pest and disease transmission. It is a vector of rabies, scabies, Echinococcus multilocularis, Toxocara canis, and Trichinella spp. [64]. According to Laurimaa et al. [65], raccoon dogs can be infected with at least 32 species of helminths, many of which are zoonotic. In Poland, the raccoon dog is the second-most important vector (after the red fox) of rabies among wild animals [66]. Some of the parasites transmitted by this species can be highly hazardous to human health, e.g., Echinococcus multilocularis or Trichinella spp. [67,68]. It is thought that it may be harmful to native birds and frogs, but hard evidence for this is difficult to obtain. Potentially, the species poses a threat to ground-nesting waterbirds, e.g., Anas platyrhynchos, Fulica atra, Anser anser, and the strictly protected Somateria mollissima, as well as the strictly protected reptile Emys orbicularis [62].
The raccoon dog is an omnivore, with its diet varying by area and season [69]. In most parts of its distribution, small rodents—voles, mice, and shrews—make up the majority of its diet in all seasons [69,70]. Frogs, lizards, invertebrates, birds, and their eggs are also frequently consumed [71]. Although the raccoon dog has been present in the Polish fauna for 70 years, little is known about its interactions with native species of predatory mammals of similar size, occupying similar trophic niches, such as the red fox (Vulpes vulpes) and European badger (Meles meles). Borowski [43] reported from the Bialowieża Primeval Forest that the overlap between the trophic niches of the European badger and raccoon dog, measured by the Pianka index [72], was 0.35 (range: from 0—niches completely disconnected to 1—niches identical). Such a low similarity index suggests that there is no trophic competition between European badgers and raccoon dogs. Therefore, fluctuations in the abundance of one species do not affect the population dynamics of the other.
According to Jędrzejewska and Jędrzejewski [63], the red fox, European badger, and raccoon dog do not compete among themselves for food resources because they have rather different trophic niches [63,73,74]. On the other hand, raccoon dogs may compete with semi-aquatic species such as American mink and Eurasian otter (Lutra lutra), where the diet similarities are 38% and 33%, respectively [63]. In the wild, raccoon dogs most often fall prey to wolves (Canis lupus) and domestic dogs (Canis familiaris) [75].
Raccoon dogs commonly make use of badger setts [76], thus benefitting from the presence of European badgers. The habit of using European badger setts has probably facilitated the invasion of raccoon dogs in Europe [76], as they provide shelter from cold and predation [58].
3.3. Northern Raccoon
The northern raccoon originates from North America, where its distribution extends from southern Canada, right across the USA and Central America as far as Panama [77]. It was introduced to Europe in the 20th century, initially in Germany (1930s), and then in Russia (1936) and Belarus (1954) [78].
The first records of wild racoons in Poland appeared in the mid-20th century. However, for a long time these were restricted to single animals. The first thriving population of raccoons was recorded in the 1990s. Numbers of racoons have been rising in recent decades, and, according to information from the Forest Data Bank, hunting harvests of raccoons have reached ~2300 individuals [79]. According to the estimates in this data bank, there are more than 8000 raccoons in Poland. However, the species is not monitored, and estimates are based on hunting harvest data.
One can divide the literature relating to raccoons in Poland into three main types: reviews presenting current knowledge of the species and its potential threat to the fauna and flora of Poland (mainly in Polish [61,80,81,82], research covering the distribution and ecology of raccoons in Poland (little work has been conducted in this respect) [83,84,85,86], and research on the role of raccoons as hosts for parasites [87,88,89,90].
Raccoons migrated to Poland mainly from Germany [83]. They are present across the whole country, but the highest concentrations are in the west; there are far fewer in central and eastern Poland [79,91]. Raccoons have been recorded in National Parks (NP), such as the Warta Mouth NP, Roztocze NP, Słowiński NP, Drawa NP, Gorce NP, Wielkopolski NP, and Wolin NP, as well as in the ornithologically important Milicz Ponds and Lake Łuknajno [83,84,92,93,94].
The extents of raccoon home ranges in Poland depend on the habitat type and abundance of food. They are small in suburban areas (1.5–2.4 km2) but larger in wetlands (on average 4.4 or 8.4 km2, depending on the season). Racoon home ranges have been found to overlap in both types of habitat, which suggests a high abundance of these animals thriving in these areas [78].
Raccoons are omnivorous, with a diet diversified across seasons and habitats. In the Warta Mouth NP, 34% of their diet consisted of rodents. Less frequently taken were birds, amphibians, fish (15%, 13%, and 12%, respectively), insects, and carcasses of large mammals [95]. Bartoszewicz et al. [78] reported the same proportion of rodents and birds in the raccoon’s diet, along with a high percentage of insects (34%), carcasses of large mammals (10%), and other vertebrates. Studies on the potential impact of raccoons on Polish fauna were conducted in the Milicz Ponds reserve [96] and in the Nietoperek nature reserve in western Poland [86]. According to Ręk [96], who studied coots Fulica atra, the impact of raccoons and American mink on the species was marginal compared to predation by hooded crows (Corvus cornix). On the other hand, Cichocki et al. [86], found that raccoons in the Nietoperek nature reserve preyed on bats hibernating in underground tunnels, and that bat remains were present in 96% of scats. To our knowledge, this is so far the only study documenting the racoon’s direct negative impact on fauna in Poland.
Raccoons are reservoirs of many pathogens and parasites; therefore, they can pose a threat to native fauna and humans. One of the most dangerous parasites spread by raccoons is Baylisascaris procyonis, which can cause neurological diseases, eye diseases, or even death in humans [97], but its prevalence in Polish individuals was found to be relatively low, not exceeding 4% [78,87,88]. The prevalence of this parasite appears to be much higher in Germany, where B. procyonis is widespread [98,99]. Several studies have been carried out to detect the presence of parasites in Polish raccoons; in addition to B. procyonis, the following parasites have been found: the phylum Acanthocephala, Ancylostoma spp., Capillaria spp., Cryptosporidium sp., Echinostoma sp., Mesocestoides spp., Placoconus lotoris, Spirocerca lupi, Strongyloides procyonis, Toxoplasma gondii, Trichinella pseudospiralis, and Trichinella spiralis [78,87,88,89,90,100]. However, the diversity of certain parasites in some cases was low, and the prevalence was less than in native or other invaded areas [87].
4. Artiodactyla
Five species of the order Artiodactyla are considered non-native to Poland, two of which are already extinct (the wapiti and the Siberian roe deer). The wapiti historically originated in North America, where its range covered territory from Mexico to Canada. Its current distribution covers much smaller territories in North America, owing to the earlier extirpation of the species [101]. Several attempts to introduce the wapiti for hunting purposes in Poland began in 1861, but none of those individuals survived for any length of time [102].
As far as the Siberian roe deer is concerned, one source points to its introduction to Poland in the early 20th century for hunting purposes, but there have never been any confirmed sightings of the species in the wild [103]. However, there are several interesting studies regarding the detection of Siberian roe deer mtDNA in the Polish roe deer population [104,105,106]. Their authors point out that this is the result of ancient hybridization at a time when the distribution of Siberian roe deer also included central Europe, rather than of its recent introductions.
4.1. Mouflon
The mouflon was first introduced to Poland in 1902, and more introductions followed in later decades, usually for hunting purposes. The modern populations of mouflons in Europe are descendants of individuals from two European islands—Corsica and Sardinia. However, the primary origin of the species in Europe is not clear, and a number of different theories are mentioned in the literature (migration of wild individuals from Asia, a feral form of previously domesticated sheep, or hybridization) [107]. In recent years, the number of mouflons in Poland has fluctuated around 3000 individuals, although hunting yields have increased slightly (Figure 1) [108]. Their distribution is insular and concentrated around the regions where they were introduced [107,109].
According to Nowakowski et al. [110], who summarized knowledge relating to mouflons in the Province of Lower Silesia, the species is divided into smaller, isolated, and rather sedentary subpopulations. In one of these subpopulations, changes in the development of horns leading to their ingrowth into the skull causing death in males have been reported. The reason for this phenomenon is the high level of inbreeding of the region’s mouflon population [110]. In areas with a high density of mouflon populations, incidents of male mouflons inseminating domestic sheep have been recorded. The high frequency of such incidents can lower the breeding value of domestic sheep [110,111].
Mouflons adversely affect limestone and neutrophilic rock habitats in that they mechanically damage the pioneer vegetation growing there [112]. There are records of these negative influences on habitats in some regions, including Natura 2000 areas, and on rare species such as Chamaecytisus supinus, Festuca pallens, Asplenium septentrionale, Digitalis grandiflora, and Jovibarba sobolifera [113]. Little is known about the impact of mouflons on other herbivores in Poland. It has been reported that, in the Czech Republic, where the climatic conditions are similar, mouflons compete with deer for food [113,114]. Mouflons are not adapted to the Polish climate and need human assistance to survive [113,115,116]. Hence, opinions diverge regarding its presence and “breeding” in Poland [113].
There have been several studies on parasites in mouflons in Poland. The following taxa and groups with a high prevalence were recorded: Muellerius capillaris, Eimeria spp., and intestinal nematodes [117],along with two other taxa with a lower prevalence, namely, Trichostrongylus sp. and Trichuris ovis [118]. Pacoń [119] and Pacoń et al. [120] reported the following other parasites in mouflons: Dicrocoelium dendriticum, Neoascaris sp., Trichocephalus sp., Strongyloides sp., Protostrongylus kochi, Dictyocaulus viviparus, Dictyocaulus filaria, Cystocaulus nigrescens, and Dicrocoelium dendriticum. In addition, mouflons can be a source of Lyme disease and salmonella infections in pets and farm animals [121,122].
4.2. Fallow Deer
Fallow deer have been a component of Polish ecosystems for several centuries. The first observations or introductions of this species in the country took place ca. 1250. However, it was not until the 19th century that large-scale introductions took place [123,124]. The species originates in southwestern Asia; however, as a result of numerous introductions, its current distribution covers North and South America, Africa, Australia, and New Zealand [125]. In Poland, it is widespread across the whole country with higher densities in the west and patches of high density in some other regions [123,126]. In the Białowieża Primeval Forest (the part now in Poland), no fallow deer were observed after 1920 because they had been eradicated by poachers and soldiers [123,127]. In 2021, there were almost 35,000 fallow deer in Poland [108]. The recent moderate increase in the hunting harvest of this species may be indicative of an increase in its population size (Figure 1).
According to Borkowski and Pudełko [128], the average home range of fallow deer in the regions studied covered from 2.06 km2 for females to 9.75 km2 for males. The most common habitats chosen by this species were grasslands, thickets, and forests. Obidziński et al. [129] compared the diets of fallow deer, roe deer (Capreolus capreolus) and red deer (Cervus elaphus) during autumn and winter in northern Europe. They indicated that competition between these species for food was possible, especially when numbers of fallow deer were increasing and during periods when food was scarce (autumn and winter in northern Europe) [129].
Several papers address the topic of parasites in fallow deer. Cisek et al. [130] found two nematodes in fallow deer feces, Elaphostrongylus cervi (prevalence ~59%) and Varestrongylus sagittatus (prevalence ~47%) (similar to [131]), whereas, in another region, Kowal et al. [132] failed to detect any lungworms. Furthermore, 12 parasite species of gastrointestinal nematodes were detected in the feces [131]. Burliński et al. [133] stated that the most common parasites in fallow deer feces were Eimeria sp., Chabertia sp., Haemonchus sp., Trichostrongylus sp., and Ostertargia sp. (with a prevalence between 10% and 20%). The differences in prevalence in the above studies reflect the variation in prevalence in different regions of Poland (e.g., red deer [132]). Szczurek et al. [134] identified three arthropod species of ectoparasites with a higher prevalence: Lipoptena cervi (76%), Ixodes ricinus (29%), and Damalinia meyeri (7%).
4.3. Sika Deer
The sika deer, native to East Asia, was introduced in 1910-1911 for hunting purposes in two regions now in Poland. One was Kobiór (Province of Silesia) in the south, and the other was Tolkmicko (Province of Warmia-Masuria) in the north (at that time, both regions belonged to the Kingdom of Prussia). The species continues to thrive in both as isolated populations. However, the sika deer is known to migrate and is slowly colonizing more distant areas [135,136,137]. The northern population consists of 250–300 animals, whereas there are around 30 in the southern population [138]. The hunting harvest of this species has decreased in previous years but is quite stable (Figure 1). According to the Atlas of Mammals of Poland, sika deer have been spotted in two regions some considerable distance away from the abovementioned populations [136]: a single individual near the town of Pisz and over a dozen near the Warta Mouth NP. They are believed to be the result of escapes or intentional releases from breeding in these areas [139].
The age ratio, sex ratio, structure, and spatial organization of the sika deer population near Kobiór were documented in 1966–1979 [140]. At that time, 233 animals were recorded with females being dominant (the ratio of males to females was 38:62) and twice as many calves as juveniles and adults. The largest herd consisted of 12 individuals, but single animals, pairs, or groups of three were most often sighted. The preferred habitat was moist mixed coniferous forest [140]. On the basis of carcass and antler measurements in the northern population, Janiszewski [141] concluded that the population was of good quality. Sika deer pose a potential threat to native populations of red deer, as there is a real possibility of hybridization, already documented in Poland [142].
Parasites of sika deer in Poland were studied long ago by Dróżdż [143], who found 12 species in 13 sika deer, of which Spiculopteragia spiculoptera was the most abundant (10 infected individuals), followed by Spiculopteragia asymetrica (n = 6), Rinadia mathevossiani (n = 4), and Cooperia pectinata (n = 4).
5. Rodentia
Five of the 31 species of rodents currently found in Poland are non-native: the house mouse, brown rat, house rat, muskrat, and coypu. There is also one extinct species, the American beaver, the appearance of which in the wild was probably due to an escape from a breeding farm near the town of Morąg in 1927 or 1932. However, research conducted after 1970 detected only Eurasian beavers in the region; thus, the American beaver is considered extinct in Poland [144,145].
5.1. House Mouse
In Poland, the house mouse is considered an alien species of undetermined population status [146]. The original range of this species included the steppe and semi-desert zones of Asia and Africa, from Japan in the east to western Africa in the west [147]. In Poland, it is currently found throughout the country [148]. The house mouse is thought to have appeared in central and northern Europe during the Bronze Age [149]. Excavation material dated to the Holocene indicates the presence of mice in present-day Belarus [150]. In Austria, house mice were present in the Bronze Age [151]. By the 19th century, they were already widespread throughout that country. In the 19th century, the house mouse was recorded in Poland’s capital, Warsaw, and in the south of the country (Podtatrze), where it was said to be abundant [152] It is a highly plastic species that quickly adapts to new, often harsh habitats (it has been found in mines, for example) [153]. The house mouse avoids high altitudes; the highest elevation in Poland where it has been found is 1500 m above sea level, in the Murowaniec mountain hostel near the Hala Gąsienicowa meadow in the Tatra Mountains [154]. The species spreads numerous diseases, as well as destroys food supplies and a variety of materials (paper, cloth, etc.), which it uses to build its nests [147].
5.2. Muskrat
In Poland, the muskrat is considered a potentially invasive species, but its numbers are declining [155]. In 1905, several animals from the USA (probably belonging to the subspecies O. z. zibethicus) were brought to the vicinity of Prague (Czech Republic) for breeding purposes. Some of these animals escaped and began to spread, migrating an estimated distance of 25 km per year. In 1924, it turned up within the present-day borders of Poland. By 1958, the species had colonized the whole of Poland. However, the 1980s saw a huge decline in the number of muskrats in Poland, such that, in many localities, it became completely extinct [156,157]. The occurrence of muskrats was monitored at 1554 localities in central and eastern Poland in 1996–1998 and 2007 [33]. Between these two periods, the frequency of muskrat records at the same localities decreased from 44% to a mere 7%. This sharp fall in the species’ abundance is corroborated by data on muskrat hunting in 1981–2017 [158,159]. For example, 9400 animals were harvested in the 2002–2003 season, in contrast only 1700 in 2016–2017 (Figure 1). About 60% of these animals came from the provinces of southern Poland [159].
At present, the muskrat is found throughout the country, most numerously in regions with a dense network of rivers and water bodies [155,160]. However, even though the muskrat’s range in Poland is slowly recovering, it is still considered a receding species, as its abundance is in decline [159]. Predation by American mink is thought to be the main reason for the diminishing numbers of muskrats, although other factors (food, parasites, and disease) cannot be ruled out [158].
Damage caused by muskrats is primarily associated with their digging burrows in riverbanks and in dykes around ponds and along rivers. Sometimes muskrats build their nests in drainage pipes, causing them to clog up, thus blocking the flow of water [155]. In addition to plant food, muskrats feed on vertebrates and aquatic invertebrates, sometimes posing a threat to endangered species like clams. Predation of mussels can reduce the abundance of fish, whose life cycle depends on the presence of suitable mollusk species, such as Rhodeus amarus roselle, in the water body.
Muskrats are reservoirs of various parasites, with the tapeworm Echinococcus multilocularis being the biggest threat. Up to 28% of the population can be infected. Since the muskrat is a prey item of the red fox and raccoon dog, infected rodents are a source of infection in mammalian predators [159]. Until 1934, the muskrat was bred for fur in Poland; however, after that year, this fur farming ceased. Currently, the muskrat is not a farm animal according to Polish legislation, but it is on the list of game animals. It can be hunted from August 11 to April 15, as well as all year round in the vicinity of fish farms [159].
5.3. House Rat
The house rat is considered an alien species with an unknown population status in Poland [161]. It originated in southeastern Asia [147]. It appeared in the territory of present-day Poland in the Late Bronze Age, and its abundance increased markedly after World War II. Twenty-one localities of this species were known after 1945: on the Baltic Coast, in the Pomeranian Lake District, in Upper and Lower Silesia, in the Sudety Mountains, and in Podlasie (eastern Poland) [147]. In 2005, house rats were found at only seven localities in a small area along the River Oder near the border with Germany (southwestern part of Lubuskie Province) [162,163].
The Atlas of Mammals of Poland [164] shows that, at present, the house rat also occurs in western Pomerania and Silesia. The small population in the Oder River basin is likely to remain at the same level. As a result of the decline in river transport, the influx of individuals from outside has diminished. Contemporary data indicate that the population is largely isolated [162]. At the same time, small, isolated populations of the species in cities where it was once found, e.g., Wroclaw, Opole, and large port cities, may still be extant.
At present, the house rat is a disappearing species in both Poland and Europe. This is mainly due to modern and effective methods of its elimination and changes in construction technologies. One factor favoring its increased abundance and wider distribution in the coming decades may be the progressive warming of the climate [162]. Like the brown rat, it is a vector of many diseases, causing food losses and damage resulting from the destruction of products and economic property [161].
5.4. Brown Rat
In Poland, the brown rat is considered an alien species with an undetermined population status [165]. It probably originated in northeastern Asia. It has spread right around the world in company with humans. Its expansion into Europe can probably be dated to the 17th–18th centuries, although some data indicate that it was earlier [147]. In Poland, it is a widely distributed species [166]. The brown rat is a vector of many diseases, causing food losses and damage resulting from the destruction of products and economic property [165].
5.5. Coypu
The coypu in Poland occurs mainly in Silesia and Lower Silesia, but single animals have also been recorded in western Poland. It has never been sighted anywhere in the east of the country [167]. This rodent was first brought to Poland as a farm animal from Argentina in 1926. Farming of this species resumed after World War II. Soon, there appeared the first reports of coypu living in the wild, one of which was the sighting of an individual in the Milicz Ponds reserve [168]. However, these animals were very vulnerable to the harsh Polish winters; thus, they did not form a permanent wild population. Currently, as a consequence of climate warming and the recent series of mild winters, coypu are more likely to survive, sych that observations of them living in ponds or rivers, even near human habitations, are becoming more frequent. Specific sightings include those at a pond in the Byczyna district of Jaworzno (where they are now extinct or have been extirpated), in the Rivers Ruda and Niacin in the town of Rybnik, or in Lower Silesia (press reports [169,170,171]).
6. Other Species
The European rabbit (Lagomorpha) has been a component of the Polish fauna since 1860, following which its numbers have fluctuated considerably [172]. Over the past 150 years, numerous introductions have been made for hunting purposes, and its current populations in Poland represent the eastern limit of the species range. They continue to be introduced into Poland every year [172,173,174,175]. To the best of our knowledge, no studies have been published on the impact of wild European rabbits on Polish wildlife.
7. A Brief Note on the Golden Jackal
There are several recent records of the golden jackal (Canis aureus) in Poland [176,177,178,179]. However, Trouwborst et al. [180] stated that this species cannot be considered an alien in Poland (or in other countries in the region), as its presence is due to natural expansion and not human activities (see CBD COP 6 Decision VI/23 definitions in Section 2) [11]. A similar approach is taken in Polish studies [176,179,181]; accordingly, this species is not included in our review.
8. Conclusions
Some non-native species, such as the raccoon dog or fallow deer, are widespread in Poland and may have a major impact on the country’s ecosystems and biodiversity, e.g., as a result of potential competition with native species, hybridization, or acting as a reservoir for parasites. There are many sources of information on these alien species, e.g., the raccoon dog and raccoon, but knowledge of their impact on native biota remains very limited. This situation can be exemplified by the mouflon; numerous sources provide information on its history in Poland, but little research has been conducted regarding its impact on native wildlife. Therefore, more research is needed to examine the impact of all these nonindigenous species on Polish wildlife in order to be able to respond adequately to their presence and expansion in Polish ecosystems and to make sound decisions regarding their management.
As the populations of non-native species recorded in recent decades have fluctuated significantly, they need to be monitored. In addition, given ongoing climate changes, the expansion of some species, e.g., coypu, may intensify. Invasive species are becoming a serious problem, but there is little reliable information on the abundance of many of them. We, therefore, believe that hunting harvests are at present the best available indicator of their population dynamics. An increase in the population size of a species usually implies an increase in its hunting harvest, and vice versa (see Figure 1).
Lastly, it is worth noting that non-native species interact with each other. The expansion of new alien species may have a significant influence on those already present and enhance or limit their population growth: American mink and muskrat are cases in point here. Such interactions are important and can affect the management of the species, underlining the importance of appropriate monitoring.
Author Contributions
Conceptualization, A.D.; methodology, A.D., H.W., M.M. and M.Z.-D.; resources, A.D., H.W., M.M. and M.Z.-D.; writing—original draft preparation, A.D., H.W., M.M. and M.Z.-D.; writing—review, A.D., H.W, M.M. and M.Z.-D.; writing—editing, A.D. and H.W. All authors read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- DAISIE. Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-8279-5. [Google Scholar]
- Kolbe, J.J.; Glor, R.E.; Schettino, L.R.; Lara, A.C.; Larson, A.; Losos, J.B. Multiple Sources, Admixture, and Genetic Variation in Introduced Anolis Lizard Populations. Conserv. Biol. 2007, 21, 1612–1625. [Google Scholar] [CrossRef]
- Lucek, K.; Roy, D.; Bezault, E.; Sivasundar, A.; Seehausen, O. Hybridization between Distant Lineages Increases Adaptive Variation during a Biological Invasion: Stickleback in Switzerland. Mol. Ecol. 2010, 19, 3995–4011. [Google Scholar] [CrossRef]
- Kolbe, J.J.; Glor, R.E.; Rodríguez Schettino, L.; Lara, A.C.; Larson, A.; Losos, J.B. Genetic Variation Increases during Biological Invasion by a Cuban Lizard. Nature 2004, 431, 177–181. [Google Scholar] [CrossRef]
- Estoup, A.; Ravignné, V.; Hufbauer, R.; Vitalis, R.; Gautier, M.; Facon, B. Is There a Genetic Paradox of Biological Invasion? Annu. Rev. Ecol. Evol. Syst. 2016, 47, 51–72. [Google Scholar] [CrossRef]
- Simberloff, D. How Much Information on Population Biology Is Needed to Manage Introduced Species? Conserv. Biol. 2003, 17, 83–92. [Google Scholar] [CrossRef]
- Abdelkrim, J.; Pascal, M.; Calmet, C.; Samadi, S. Importance of Assessing Population Genetic Structure before Eradication of Invasive Species: Examples Form Insular Norway Rat Populations. Conserv. Biol. 2005, 19, 1509–1518. [Google Scholar] [CrossRef]
- Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species; Official Journal of the European Union, European Union: Italy, The Netherlands, 2014; Volume 317.
- Instytut Ochrony Przyrody PAN. Available online: https://www.iop.krakow.pl/ (accessed on 19 December 2022).
- The Conference of the Parties 6 Decision VI/23; Convention on Biological Diversity (CBD): The Hague, The Netherlands, 2002.
- Inwazyjne Gatunki Obce (IGO)—Generalna Dyrekcja Ochrony Środowiska—Portal Gov.pl. Available online: https://www.gov.pl/web/gdos/inwazyjne-gatunki-obce3 (accessed on 10 January 2023).
- Document Search—Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 19 December 2022).
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 19 December 2022).
- Google Scholar. Available online: https://scholar.google.com/ (accessed on 19 December 2022).
- Google. Available online: https://www.google.com/ (accessed on 19 December 2022).
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/114 (accessed on 27 November 2022).
- Zestawienie Danych Sprawozdawczości Łowieckiej 2019 Rok; Stacja Badawcza PZŁ Czempiń: Czempiń, Poland, 2019.
- Kamieniarz, R.; Panek, M. Zwierzęta Łowne w Polsce na przełomie XX i XXI Wieku = Game Animals in Poland at the Turn of the 20th and 21st Century; Polski Związek Łowiecki Stacja Badawcza: Czempiń, Poland, 2008; ISBN 978-83-904442-9-1. [Google Scholar]
- Brzeziński, M.; Marzec, M. The Origin, Dispersal and Distribution of the American Mink Mustela Vison in Poland. Acta Theriol. 2003, 48, 505–514. [Google Scholar] [CrossRef]
- Zalewski, A.; Michalska-Parda, A.; Bartoszewicz, M.; Kozakiewicz, M.; Brzeziński, M. Multiple Introductions Determine the Genetic Structure of an Invasive Species Population: American Mink Neovison Vison in Poland. Biol. Conserv. 2010, 143, 1355–1363. [Google Scholar] [CrossRef]
- Zalewski, A.; Michalska-Parda, A.; Ratkiewicz, M.; Kozakiewicz, M.; Bartoszewicz, M.; Brzeziński, M. High Mitochondrial DNA Diversity of an Introduced Alien Carnivore: Comparison of Feral and Ranch American Mink Neovison Vison in Poland. Divers. Distrib. 2011, 17, 757–768. [Google Scholar] [CrossRef]
- Mucha, A.; Zatoń-Dobrowolska, M.; Moska, M.; Wierzbicki, H.; Dziech, A.; Bukaciński, D.; Bukacińska, M. How Selective Breeding Has Changed the Morphology of the American Mink (Neovison Vison) – A Comparative Analysis of Farm and Feral Animals. Animals 2021, 11, 106. [Google Scholar] [CrossRef]
- Brzeziński, M.; Żmihorski, M.; Zarzycka, A.; Zalewski, A. Expansion and Population Dynamics of a Non-Native Invasive Species: The 40-Year History of American Mink Colonisation of Poland. Biol. Invasions 2019, 21, 531–545. [Google Scholar] [CrossRef]
- Niemczynowicz, A.; Brzeziński, M.; Zalewski, A. Aliens Attack—Population Dynamics and Density Control of American Mink Neovison Vison in Four National Parks in Poland. In Proceedings of the 8th European Vertebrate Pest Management Conference, Berlin, Germany, 26–30 September 2011; Julius-Kühn-Archiv: Quedlinburg, Germany, 2011; p. 432. [Google Scholar]
- Krawczyk, A.; Bogdziewicz, M.; Czyz, M. Diet of the American Mink Neovison Vison in an Agricultural Landscape in Western Poland. Folia Zool. Brno 2013, 62, 304–310. [Google Scholar] [CrossRef]
- Hurníková, Z.; Kołodziej-Sobocińska, M.; Dvorožňáková, E.; Niemczynowicz, A.; Zalewski, A. An Invasive Species as an Additional Parasite Reservoir: Trichinella in Introduced American Mink (Neovison Vison). Vet. Parasitol. 2016, 231, 106–109. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M.; Brzeziński, M.; Niemczynowicz, A.; Zalewski, A. High Parasite Infection Level in Non-Native Invasive Species: It Is Just a Matter of Time. Ecography 2018, 41, 1283–1294. [Google Scholar] [CrossRef]
- Brzeziński, M.; Natorff, M.; Zalewski, A.; Żmihorski, M. Numerical and Behavioral Responses of Waterfowl to the Invasive American Mink: A Conservation Paradox. Biol. Conserv. 2012, 147, 68–78. [Google Scholar] [CrossRef]
- Brzeziński, M.; Marzec, M.; Żmihorski, M. Spatial Distribution, Activity, Habitat Selection of American Mink (Neovison Vison) and Polecats (Mustela putorius) Inhabiting the Vicinity of Eutrophic Lakes in NE Poland. Folia Zool. Brno 2010, 59, 183–191. [Google Scholar] [CrossRef]
- Brzeziński, M.; Zarzycka, A.; Diserens, T.A.; Zalewski, A. Does the American Mink Displace the European Polecat? A Need for More Research on Interspecific Competition between Invasive and Native Species. Eur. J. Wildl. Res. 2021, 67, 64. [Google Scholar] [CrossRef]
- Zalewski, A.; Szymura, M.; Kowalczyk, R.; Brzeziński, M. Low Individual Diet Variation and High Trophic Niche Overlap between the Native Polecat and Invasive American Mink. J. Zool. 2021, 314, 151–161. [Google Scholar] [CrossRef]
- Romanowski, J.; Karpowicz, K. Zmiany w występowaniu piżmaka Ondatra zibethicus w centralnej i wschodniej Polsce w latach 1996–2007. Stud. Ecol. Bioethicae 2013, 1, 49–61. [Google Scholar] [CrossRef]
- Brzeziński, M.; Ignatiuk, P.; Żmihorski, M.; Zalewski, A. An Invasive Predator Affects Habitat Use by Native Prey: American Mink and Water Vole Co-Existence in Riparian Habitats. J. Zool. 2018, 304, 109–116. [Google Scholar] [CrossRef]
- Kloskowski, J. Human–Wildlife Conflicts at Pond Fisheries in Eastern Poland: Perceptions and Management of Wildlife Damage. Eur. J. Wildl. Res. 2011, 57, 295–304. [Google Scholar] [CrossRef]
- Manikowska-Ślepowrońska, B.; Szydzik, B.; Jakubas, D. Determinants of the Presence of Conflict Bird and Mammal Species at Pond Fisheries in Western Poland. Aquat. Ecol. 2016, 50, 87–95. [Google Scholar] [CrossRef]
- Kauhala, K.; Saeki, M. Raccoon Dogs: Finnish and Japanese Raccoon Dogs—On the Road to Speciation? In The Biology and Conservation of Wild Canids; Macdonald, D.W., Sillero-Zubiri, C., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 217–226. ISBN 978-0-19-851556-2. [Google Scholar]
- Włodek, K.; Krzywiński, A. Zu Biologie und Verhalten des Marderhundes (Nyctereutes procyonoides) in Polen. Z. Für Jagdwiss. 1986, 32, 203–215. [Google Scholar] [CrossRef]
- Jȩdrzejewski, W.; Jȩdrzejewska, B. Predation on Rodents in Białowieża Primeval Forest, Poland. Ecography 1993, 16, 47–64. [Google Scholar] [CrossRef]
- Goszczyński, J.; Skoczyńska, J. Density Estimation, Family Group Size and Recruitment in a Badger Population near Rogów (Central Poland). Misc. Zool. 1996, 19, 27–33. [Google Scholar]
- Goszczynski, J. Fox, Raccoon Dog and Badger Densities in North Eastern Poland. Acta Theriol. 1999, 44, 413–420. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Zalewski, A.; Jędrzejewska, B.; Jȩdrzejewski, W. Social Relationships in Raccoon Dogs; Institute of Vertebrate Biology, Academy of Sciences of the Czech Republik: Brno, Czech Republic, 2003; p. 114. [Google Scholar]
- Borowski, Z. Interakcje pomiędzy trzema gatunkami ssaków drapieżnych: Jenotem borsukiem i lisem—Konkurencja czy koegzystencja? Sylwan 2006, 150, 58–66. [Google Scholar]
- Grabińska, B. Uwarunkowania Naturalne i Antropogeniczne Rozmieszczenia Ssaków Łownych w Polsce; IGiPZ PAN: Warszawa, Poland, 2011; ISBN 978-83-61590-18-7. [Google Scholar]
- Budny, M.; Bresiński, W.; Kamieniarz, R.; Kolanoś, B.; Mąka, H.; Panek, M. Sytuacja Zwierząt Łownych w Polsce w Roku Łowieckim 2010/2011 (Wyniki Monitoringu); Biuletyn Stacji Badawczej w Czempiniu Nr 8 2011; Stacja Badawcza—OHZ PZŁ w Czempiniu: Czempiń, Poland, 2011. [Google Scholar]
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/103 (accessed on 27 November 2022).
- Horecka, B.; Jakubczak, A.; Ślaska, B.; Jeżewska-Witkowska, G. Raccoon Dog (Nyctereutes procyonoides) Phylogeography Including the Polish Population: Local and Global Aspects. Eur. Zool. J. 2022, 89, 641–652. [Google Scholar] [CrossRef]
- Slaska, B.; Zieba, G.; Rozempolska-Rucinska, I.; Jezewska-Witkowska, G.; Jakubczak, A. Evaluation of Genetic Biodiversity in Farm-Bred and Wild Raccoon Dogs in Poland. Folia Biol. 2010, 58, 195–199. [Google Scholar] [CrossRef]
- Slaska, B.; Grzybowska-Szatkowska, L. Analysis of the Mitochondrial Haplogroups of Farm and Wild-Living Raccoon Dogs in Poland. Mitochondrial DNA 2011, 22, 105–110. [Google Scholar] [CrossRef]
- Bugno-Poniewierska, M.; Wroński, M.; Potocki, L.; Pawlina, K.; Wnuk, M.; Jeżewska-Witkowska, G.; Słota, E. The Polymorphism of Cytogenetic Markers in the Farm and Wild-Living Raccoon Dog (Nyctereutes procyonoides)/Polimorfizm Markerów Cytogenetycznych U Jenota (Nyctereutes procyonoides) W Populacjach Hodowlanych I Dziko Żyjących. Ann. Anim. Sci. 2013, 13, 701–713. [Google Scholar] [CrossRef]
- Kasperek, K.; Horecka, B.; Jakubczak, A.; Ślaska, B.; Gryzińska, M.; Bugno-Poniewierska, M.; Piórkowska, M.; Jeżewska-Witkowska, G. Analysis of Genetic Variability in Farmed and Wild Populations of Raccoon Dog (Nyctereutes procyonoides) Using Microsatellite Sequences. Ann. Anim. Sci. 2015, 15, 889–901. [Google Scholar] [CrossRef]
- Slaska, B.; Zieba, G.; Rozempolska-Rucinska, I.; Jezewska-Witkowska, G.; Nisztuk, S.; Horecka, B.; Zon, A. Mitochondrial DNA Haplotypes Are Associated with Performance Traits in Raccoon Dogs. Anim. Sci. Pap. Rep. 2016, 34, 293–302. [Google Scholar]
- Nisztuk-Pacek, S.; Slaska, B.; Zieba, G.; Rozempolska-Rucinska, I. Two Mitochondrial Genes Are Associated with Performance Traits in Farmed Raccoon Dogs (Nyctereutes procyonoides). Czech J. Anim. Sci. 2018, 63, 110–118. [Google Scholar] [CrossRef]
- Pasławski, T. Łowiectwo dla Leśników i Myśliwych; Wydawnictwo Świat: Warszawa, Poland, 1994; ISBN 83-09-00308-0. [Google Scholar]
- Grabińska, B. Zmienność Przestrzenna i Czasowa Rozmieszczenia Ssaków Łownych Polski. PAN IGiPZ: Warszawa, Poland, 2007; ISBN 978-83-87954-89-6. [Google Scholar]
- Dehnel, A. Nowy Ssak Dla Fauny Polskiej Nyctereutes Procynoides (Gray). Chrońmy Przyr. Ojczystą 1956, 12, 17–21. [Google Scholar]
- Nowak, E.; Pielowski, Z. Jenot w Polsce. Low. Pol. 1964, 20, 3–4. [Google Scholar]
- Kowalczyk, R.; Zalewski, A. Adaptation to Cold and Predation—Shelter Use by Invasive Raccoon Dogs Nyctereutes procyonoides in Białowieża Primeval Forest (Poland). Eur. J. Wildl. Res. 2011, 57, 133–142. [Google Scholar] [CrossRef]
- Osten-Sacken, N.; Ziomek, J.; Kardynia, P.; Zgrabczynska, E. Distribution of the Raccoon Dog Nyctereutes procyonoides in Western Poland. Fragm. Faun. 2011, 54, 95–102. [Google Scholar] [CrossRef]
- Cybulska, A.; Kornacka, A.; Moskwa, B. The Occurrence and Muscle Distribution of Trichinella britovi in Raccoon Dogs (Nyctereutes procyonoides) in Wildlife in the Głęboki Bród Forest District, Poland. Int. J. Parasitol. Parasites Wildl. 2019, 9, 149–153. [Google Scholar] [CrossRef]
- Święcicka, N.; Kubacki, S.; Zawiślak, J.; Gulda, D.; Monkiewicz, M.; Drewka, M. Jenot i Szop Pracz Jako Gatunki Ekspansywne w Polsce. Przegl. Hod. 2011, 6, 10–12. [Google Scholar]
- Kowalczyk, R.; Zalewski, A.; Okarma, H. Karta Informacyjna Gatunku Jenot Nyctereutes Procyonoides Gray, 1834; Analiza stopnia inwazyjności gatunków obcych w Polsce wraz ze wskazaniem gatunków istotnie zagrażających rodzimej florze i faunie oraz propozycją działań strategicznych w zakresie możliwości ich zwalczania oraz analiza dróg niezamierzonego wprowadzania lub rozprzestrzeniania się inwazyjnych gatunków obcych wraz z opracowaniem planów działań dla dróg priorytetowych; GDOŚ: Warszawa, Poland, 2018.
- Jedrzejewska, B.; Jedrzejewski, W. Predation in Vertebrate Communities: The Białowieża Primeval Forest as a Case Study; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-642-08384-6. [Google Scholar]
- Pilarczyk, B.M.; Tomza-Marciniak, A.K.; Pilarczyk, R.; Rząd, I.; Bąkowska, M.J.; Udała, J.M.; Tylkowska, A.; Havryliak, V. Infection of Raccoon Dogs (Nyctereutes procyonoides) from Northern Poland with Gastrointestinal Parasites as a Potential Threat to Human Health. J. Clin. Med. 2022, 11, 1277. [Google Scholar] [CrossRef]
- Laurimaa, L.; Süld, K.; Davison, J.; Moks, E.; Valdmann, H.; Saarma, U. Alien Species and Their Zoonotic Parasites in Native and Introduced Ranges: The Raccoon Dog Example. Vet. Parasitol. 2016, 219, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kauhala, K.; Kowalczyk, R. Invasion of the Raccoon Dog Nyctereutes procyonoides in Europe: History of Colonization, Features behind Its Success, and Threats to Native Fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef]
- Mayer-Scholl, A.; Reckinger, S.; Schulze, C.; Nöckler, K. Study on the Occurrence of Trichinella Spp. in Raccoon Dogs in Brandenburg, Germany. Vet. Parasitol. 2016, 231, 102–105. [Google Scholar] [CrossRef]
- Kärssin, A.; Häkkinen, L.; Niin, E.; Peik, K.; Vilem, A.; Jokelainen, P.; Lassen, B. Trichinella Spp. Biomass Has Increased in Raccoon Dogs (Nyctereutes procyonoides) and Red Foxes (Vulpes vulpes) in Estonia. Parasites Vectors 2017, 10, 609. [Google Scholar] [CrossRef]
- Nasimovich, A.; Isakov, Y. Arctic Fox, Red Fox and Raccoon Dog: Distribution of Populations, Ecology and Preservation; Nauka: Moscow, Russia, 1985. [Google Scholar]
- Kauhala, K.; Helle, E.; Pietilä, H. Time Allocation of Male and Female Raccoon Dogs to Pup Rearing at the Den. Acta Theriol. 1998, 43, 301–310. [Google Scholar] [CrossRef]
- Kauhala, K.; Auniola, M. Diet of Raccoon Dogs in Summer in the Finnish Archipelago. Ecography 2001, 24, 151–156. [Google Scholar] [CrossRef]
- Pianka, E.R. The Structure of Lizard Communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Reig, S.; Jędrzejewski, W. Winter and Early Spring Food of Some Carnivores in the Białowieża National Park, Eastern Poland. Acta Theriol. 1988, 33, 57–65. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Jędrzejewska, B.; Szymura, A. Food Niche Overlaps in a Winter Community of Predators in the Białowieża Primeval Forest, Poland. Acta Theriol. 1989, 34, 487–496. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Zalewski, A.; Jędrzejewska, B.; Ansorge, H.; Bunevich, A.N. Reproduction and Mortality of Invasive Raccoon Dogs (Nyctereutes procyonoides) in the Białowieża Primeval Forest (Eastern Poland). Ann. Zool. Fenn. 2009, 46, 291–301. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Jędrzejewska, B.; Zalewski, A.; Jedrzejewski, W. Facilitative Interactions between the Eurasian Badger (Meles Meles), the Red Fox (Vulpes vulpes), and the Invasive Raccoon Dog (Nyctereutes procyonoides) in Bialowieza Primeval Forest, Poland. Can. J. Zool. 2008, 86, 1389–1396. [Google Scholar] [CrossRef]
- Lotze, J.-H.; Anderson, S. Procyon Lotor. Mamm. Species 1979, 1–8. [Google Scholar] [CrossRef]
- Bartoszewicz, M.; Okarma, H.; Zalewski, A.; Szczȩsna, J. Ecology of the Raccoon (Procyon lotor) from Western Poland. Ann. Zool. Fenn. 2008, 45, 291–298. [Google Scholar] [CrossRef]
- Bank Danych o Lasach. Available online: https://www.bdl.lasy.gov.pl/portal/tworzenie-zestawienia-rlo-en (accessed on 27 November 2022).
- Solarz, W. Drobne Inwazyjne Obce Drapieżniki w Polsce Small Invasive Alien Predators in Poland. Ann. Wars. Univ. Life Sci.-SGGW Anim. Sci. 2011, 50, 73–81. [Google Scholar]
- Okarma, H.; Zalewski, A.; Bartoszewicz, M.; Biedrzycka, A.; Jędrzejewska, E. Szop Pracz Procyon lotor w Polsce Ekologia Inwazji. Stud. I Mater. Cent. Edukac. Przyr. -Leśnej 2012, 14, 296–303. [Google Scholar]
- Felska-Błaszczyk, L.; Boniek, M.; Ławrów, N.; Seremak, B. Szop Pracz (Procyon lotor)-Gatunek Inwazyjny Obcy w Faunie Polski. Charakterystyka Gatunku, Wielkość Populacji i Mechanizmy Inwazyjności. Wiadomości Zootech. 2017, 3, 24–34. [Google Scholar]
- Biedrzycka, A.; Zalewski, A.; Bartoszewicz, M.; Okarma, H.; Jędrzejewska, E. The Genetic Structure of Raccoon Introduced in Central Europe Reflects Multiple Invasion Pathways. Biol. Invasions 2014, 16, 1611–1625. [Google Scholar] [CrossRef]
- Gabryś, G.; Nowaczyk, J.; Ważna, A.; Kościelska, A.; Nowakowski, K.; Cichocki, J. Expansion of the Raccoon Procyon lotor in Poland. Acta Biol. 2014, 21, 169–181. [Google Scholar]
- Kopij, G. Expansion of Alien Carnivore and Ungulate Species in SW Poland. Russ. J. Biol. Invasions 2017, 8, 290–299. [Google Scholar] [CrossRef]
- Cichocki, J.; Ważna, A.; Bator-Kocoł, A.; Lesiński, G.; Grochowalska, R.; Bojarski, J. Predation of Invasive Raccoon (Procyon lotor) on Hibernating Bats in the Nietoperek Reserve in Poland. Mamm. Biol. 2021, 101, 57–62. [Google Scholar] [CrossRef]
- Popiołek, M.; Szczȩsna-Staśkiewicz, J.; Bartoszewicz, M.; Okarma, H.; Smalec, B.; Zalewski, A. Helminth Parasites of an Introduced Invasive Carnivore Species, the Raccoon (Procyon lotor L.), from the Warta Mouth National Park (Poland). J. Parasitol. 2011, 97, 357–360. [Google Scholar] [CrossRef]
- Karamon, J.; Kochanowski, M.; Cencek, T.; Bartoszewicz, M.; Kusyk, P. Gastrointestinal Helminths of Raccoons (Procyon lotor) in Western Poland (Lubuskie province)—With Particular Regard to Baylisascaris procyonis. Bull. Vet. Inst. Pulawy 2014, 58, 547–552. [Google Scholar] [CrossRef]
- Leśniańska, K.; Perec-Matysiak, A.; Hildebrand, J.; Buńkowska-Gawlik, K.; Piróg, A.; Popiołek, M. Cryptosporidium Spp. and Enterocytozoon Bieneusi in Introduced Raccoons (Procyon lotor)—First Evidence from Poland and Germany. Parasitol. Res. 2016, 115, 4535–4541. [Google Scholar] [CrossRef]
- Kornacka, A.; Cybulska, A.; Popiołek, M.; Kuśmierek, N.; Moskwa, B. Survey of Toxoplasma Gondii and Neospora Caninum in Raccoons (Procyon lotor) from the Czech Republic, Germany and Poland. Vet. Parasitol. 2018, 262, 47–50. [Google Scholar] [CrossRef]
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/115 (accessed on 27 November 2022).
- Jamrozy, G. Carnivores, Even-Toed Ungulates, Lagomorphs and Large Rodents in Polish National Parks. Ann. Zool. Fenn. 2008, 45, 299–307. [Google Scholar] [CrossRef]
- Mysłajek, R.W.; Figura, M.; Stachyra, P.; Nowak, S. Stwierdzenie Szopa Pracza Procyon lotor w Roztoczańskim Parku Narodowym. Record of the Raccoon Procyon lotor in Roztocze National Park. Przegląd Przyr. 2019, 30, 116–118. [Google Scholar]
- Goc, M.; Jędro, G.; Jędro, M. Obserwacja Szopa Pracza Procyon lotor w Słowińskim Parku Narodowym Observations of the Raccoon Procyon lotor in Słowiński National Park. Przegląd Przyr. 2020, XXXI, 89–92. [Google Scholar]
- Wojtaszyn, G.; Rutkowski, T.; Lesiński, G.; Stephan, W.; Bartoszewicz, M. Soricomorphs and Rodents of the Ujście Warty National Park and the Surrounding Area. Chrońmy Przyr. Ojcz. 2015, 71, 179–191. [Google Scholar]
- Ręk, P. Are Changes in Predatory Species Composition and Breeding Performance Responsible for the Decline of Coots Fulica Atra in Milicz Ponds Reserve (SW Poland)? Acta Ornitol. 2009, 44, 45–52. [Google Scholar] [CrossRef]
- Wise, M.E.; Sorvillo, F.J.; Shafir, S.C.; Ash, L.R.; Berlin, O.G. Severe and Fatal Central Nervous System Disease in Humans Caused by Baylisascaris Procyonis, the Common Roundworm of Raccoons: A Review of Current Literature. Microbes Infect. 2005, 7, 317–323. [Google Scholar] [CrossRef]
- Rentería-Solís, Z.; Birka, S.; Schmäschke, R.; Król, N.; Obiegala, A. First Detection of Baylisascaris Procyonis in Wild Raccoons (Procyon lotor) from Leipzig, Saxony, Eastern Germany. Parasitol. Res. 2018, 117, 3289–3292. [Google Scholar] [CrossRef]
- Heddergott, M.; Steinbach, P.; Schwarz, S.; Anheyer-Behmenburg, H.E.; Sutor, A.; Schliephake, A.; Jeschke, D.; Striese, M.; Müller, F.; Meyer-Kayser, E.; et al. Geographic Distribution of Raccoon Roundworm, Baylisascaris Procyonis, Germany and Luxembourg. Emerging Infect. Dis. 2020, 26, 821. [Google Scholar] [CrossRef]
- Cybulska, A.; Skopek, R.; Kornacka, A.; Popiołek, M.; Piróg, A.; Laskowski, Z.; Moskwa, B. First Detection of Trichinella Pseudospiralis Infection in Raccoon (Procyon lotor) in Central Europe. Vet. Parasitol. 2018, 254, 114–119. [Google Scholar] [CrossRef]
- Dunn, C.D.; Wilkinson, J.E. Genetic Diversity and the Possible Origin of Contemporary Elk (Cervus canadensis) Populations in the Trans-Pecos Region of Texas. Occas. Pap. Tex. Tech Univ. Mus. 2017, 350, 1–16. [Google Scholar]
- Okarma, H.; Wierzbowska, I.; Mazurska, K. Karta Informacyjna Gatunku Wapiti Cervus canadensis Erxleben, 1777; Analiza stopnia inwazyjności gatunków obcych w Polsce wraz ze wskazaniem gatunków istotnie zagrażających rodzimej florze i faunie oraz propozycją działań strategicznych w zakresie możliwości ich zwalczania oraz Analiza dróg niezamierzonego wprowadzania lub rozprzestrzeniania się inwazyjnych gatunków obcych wraz z opracowaniem planów działań dla dróg priorytetowych; GDOŚ: Warszawa, Poland, 2018.
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/788 (accessed on 27 November 2022).
- Olano-Marin, J.; Plis, K.; Sönnichsen, L.; Borowik, T.; Niedziałkowska, M.; Jędrzejewska, B. Weak Population Structure in European Roe Deer (Capreolus capreolus) and Evidence of Introgressive Hybridization with Siberian Roe Deer (C. pygargus) in Northeastern Poland. PLoS ONE 2014, 9, e109147. [Google Scholar] [CrossRef]
- Matosiuk, M.; Borkowska, A.; Świsłocka, M.; Mirski, P.; Borowski, Z.; Krysiuk, K.; Danilkin, A.A.; Zvychaynaya, E.Y.; Saveljev, A.P.; Ratkiewicz, M. Unexpected Population Genetic Structure of European Roe Deer in Poland: An Invasion of the MtDNA Genome from Siberian Roe Deer. Mol. Ecol. 2014, 23, 2559–2572. [Google Scholar] [CrossRef]
- Świsłocka, M.; Czajkowska, M.; Matosiuk, M.; Saveljev, A.P.; Ratkiewicz, M.; Borkowska, A. No Evidence for Recent Introgressive Hybridization between the European and Siberian Roe Deer in Poland. Mamm. Biol. 2019, 97, 59–63. [Google Scholar] [CrossRef]
- Nasiadka, P.; Wajdzik, M.; Skubis, J. A Comprehensive over 100 Years History of Mouflon (Ovis musimon) in Poland: From the Promising Beginningin1902 to Questionable Future in 2014–a Case Study of Wildlife Management History. Appl. Ecol. Environ. Res. 2021, 19, 993–1017. [Google Scholar] [CrossRef]
- Rozkrut, D. Statistics Poland Statistical Yearbook of Forestry; GUS: Warszawa, Poland, 2021. [Google Scholar]
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/129 (accessed on 27 November 2022).
- Nowakowski, P.; Chudoba, K.; Piasecki, M. Muflon Europejski (Ovis Orientalis Musimon Schreber, 1782) w Ekosystemie Dolnego Śląska. Ann. Univ. Mariae Curie-Sklodowska, Zootechnica 2009, 27, 7–13. [Google Scholar] [CrossRef]
- Bobek, B.; Frąckowiak, W.; Furtek, J.; Merta, D. Integration of Introduced Mouflons with the Local Population in the Sutedy Mountains. Balk. J. Wildl. Res. 2014, 1, 82–86. [Google Scholar] [CrossRef]
- Mróz, W. Monitoring Siedlisk Przyrodniczych. Przewodnik Metodyczny. Cz I; GIOŚ: Warszawa, Poland, 2010; ISBN 978-83-61227-52-6.
- Szczęśniak, E. Obecność Muflonów Ovis Aries Musimon w Polsce—Czy to Naprawdę Konieczne? Chrońmy Przyr. Ojczystą 2011, 67, 99–117. [Google Scholar]
- Homolka, M. The Food Niches of Three Ungulate Species in a Woodland Complex. Folia Zool. (Brno) 1993, 42, 193–203. [Google Scholar]
- Warchałowski, M.; Nowakowski, P.; Dancewicz, A. Effect of Winter Conditions on Wild Ungulates Mortality in the Owl Mountains (Poland). Folia For. Pol. 2015, 57, 187–193. [Google Scholar] [CrossRef]
- Tajchman, K.; Drozd, L. Management of Hunting Animals Population as Breeding Work Part III: Hunting and Breeding Work on Introduced Fauna. Ann. Wars. Univ. Life Sci. -SGGW Anim. Sci. 2018, 57, 419–427. [Google Scholar] [CrossRef]
- Bartczak, R.; Okulewicz, A. Epizootic Situation of Mouflon Ovis Aries Musimon in Lower Silesia on the Basis of Coproscopic Examinations. Ann. Parasitol. 2014, 60, 253–258. [Google Scholar]
- Balicka-Ramisz, A.; Laurans, Ł.; Jurczyk, P.; Kwita, E.; Ramisz, A. Gastrointestinal Nematodes and the Deworming of Mouflon (Ovis Aries Musimon) from Goleniowska Forest in West Pomerania Province, Poland. Ann. Parasitol. 2017, 63, 27–32. [Google Scholar] [CrossRef]
- Pacon, J. Pasożyty Muflonów, Jeleni i Sarn z Terenu Dolnego Śląska. Ann. Parasitol. 1994, 40, 279–292. [Google Scholar]
- Pacoń, J.; Jasiński, K.; Sołtysiak, Z. Pasożyty Wewnętrzne Muflonów (Ovis musimon) z Wybranych Terenów Dolnego Śląska. Ann. Parasitol. 2007, 53, 55. [Google Scholar]
- Glinski, Z.; Zmuda, A. Zwierzęta Łowne Rezerwuarem Chorób Zakaźnych Dla Zwierząt Hodowlanych. Życie Weter. 2021, 96, 559–565. [Google Scholar]
- Martín-Atance, P.; León, L.; Candela, M.G.; Martín-Atance, P.; León, L.; Candela, M.G. Serology as an Epidemiological Tool for Salmonella Abortusovis Surveillance in the Wild-Domestic Ruminant Interface. In Salmonella - A Diversified Superbug; Kumar, Y., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-307-781-9. [Google Scholar]
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/124 (accessed on 27 November 2022).
- Solarz, W. Dama dama (Linnaeus, 1758) Daniel. In Gatunki Obce w Faunie Polski. Wyd. Internetowe; IOP PAN: Kraków, Poland, 2012. [Google Scholar]
- Ludwig, A.; Vernesi, C.; Lieckfeldt, D.; Lattenkamp, E.Z.; Wiethölter, A.; Lutz, W. Origin and Patterns of Genetic Diversity of German Fallow Deer as Inferred from Mitochondrial DNA. Eur. J. Wildl. Res. 2012, 58, 495–501. [Google Scholar] [CrossRef]
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/189 (accessed on 27 November 2022).
- Jedrzejewska, B.; Jędrzejewski, W.; Bunevich, A.N.; Milkowski, L.; Krasiński, Z.A. Factors Shaping Population Densities and Increase Rates of Ungulates in Bialowieza Primeval Forest (Poland and Belarus) in the 19th and 20th Centuries. Acta Theriol. 1997, 4, 399–451. [Google Scholar] [CrossRef]
- Borkowski, J.; Pudełko, M. Marek Forest Habitat Use and Home-Range Size in Radio-Collared Fallow Deer. Ann. Zool. Fenn. 2007, 44, 107–114. [Google Scholar]
- Obidziński, A.; Kiełtyk, P.; Borkowski, J.; Bolibok, L.; Remuszko, K. Autumn-Winter Diet Overlap of Fallow, Red, and Roe Deer in Forest Ecosystems, Southern Poland. Cent. Eur. J. Biol. 2013, 8, 8–17. [Google Scholar] [CrossRef]
- Cisek, A.; Balicka-Ramisz, A.; Ramisz, A.; Pilarczyk, B. Course and Treatment of Lungworm Infection Game Animals (Red Deer, Roe Deer, and Fallow Deer) in North—West Poland. Electron. J. Pol. Agric. Universities. Ser. Vet. Med. 2003, 6, 1–7. [Google Scholar]
- Balicka-Ramisz, A.; Pilarczyk, B.; Ramisz, A.; Cisek, A. Occurrence of Gastrointestinal and Pulmonary Nematodes of Fallow Deer (Dama dama L.) in North-West Poland. Acta Parasitol. 2005, 50, 94–96. [Google Scholar]
- Kowal, J.; Kornaś, S.; Nosal, P.; Basiaga, M.; Wajdzik, M.; Skalska, M.; Wyrobisz, A. Lungworm (Nematoda: Protostrongylidae) Infection in Wild and Domestic Ruminants from Malopolska Region of Poland. Ann. Parasitol. 2016, 62, 63–66. [Google Scholar] [CrossRef]
- Burlinski, P.; Janiszewski, P.; Anna, K.; Gonkowski, S. Parasitofauna in the Gastrointestinal Tract of the Cervids (Cervidae) in Northern Poland. Acta Vet. 2011, 61, 269–282. [Google Scholar] [CrossRef]
- Szczurek, B.; Kadulski, S. Ectoparasites on Fallow Deer, Dama dama [L.] in Pomerania, Poland. Acta Parasitol. 2004, 1, 80–86. [Google Scholar]
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/185 (accessed on 27 November 2022).
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/123 (accessed on 27 November 2022).
- Feldhamer, G.A. Cervus Nippon. Mamm. Species 1980, 1–7. [Google Scholar] [CrossRef]
- Zalewski, D. Strategia Polskiego Związku Łowieckiego w Postępowaniu z Gatunkami Obcymi w Ekosystemach Leśnych. Stud. I Mater. Cent. Edukac. Przyr. -Leśnej 2012, 4, 304–318. [Google Scholar]
- Solarz, W.; Okarma, H.; Mazurska, K. Jeleń Sika (Jeleń Wschodni) Cervus Nippon Temminck, 1838; Analiza stopnia inwazyjności gatunków obcych w Polsce wraz ze wskazaniem gatunków istotnie zagrażających rodzimej florze i faunie oraz propozycją działań strategicznych w zakresie możliwości ich zwalczania oraz Analiza dróg niezamierzonego wprowadzania lub rozprzestrzeniania się inwazyjnych gatunków obcych wraz z opracowaniem planów działań dla dróg priorytetowych; GDOŚ: Warszawa, Poland, 2018.
- Dzięciołowski R Structure and Spatial Organization of Deer Populations 1. Acta Theriol. 1979, 24, 3–21. [CrossRef]
- Janiszewski, P.; Daszkiewicz, T.; Szczepanik, A. Carcass Weight, Carcass Composition and Antler Quality of the Sika Deer (Cervus nippon) in Poland. Sylwan 2007, 1, 11–19. [Google Scholar]
- Biedrzycka, A.; Solarz, W.; Okarma, H. Hybridization between Native and Introduced Species of Deer in Eastern Europe. J. Mamm. 2012, 93, 1331–1341. [Google Scholar] [CrossRef]
- Drozdz, J. Helmintofauna Zaaklimatyzowanego w Polsce Jelenia Sika (Cervus nippon L.). Ann. Parasitol. 1963, IX (2), 133–138. [Google Scholar]
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/186 (accessed on 27 November 2022).
- Czech, A.; Janiszewski, P.; Solarz, W. Bóbr Amerykański Castor canadensis Kuhl, 1820; Analiza stopnia inwazyjności gatunków obcych w Polsce wraz ze wskazaniem gatunków istotnie zagrażających rodzimej florze i faunie oraz propozycją działań strategicznych w zakresie możliwości ich zwalczania oraz Analiza dróg niezamierzonego wprowadzania lub rozprzestrzeniania się inwazyjnych gatunków obcych wraz z opracowaniem planów działań dla dróg priorytetowych; GDOŚ: Warszawa, Poland, 2018.
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/780 (accessed on 27 November 2022).
- Kowalski, K.; Ruprecht, A.L. Myszowate—Muridae. In Klucz do Oznaczania Ssaków Polski; PWN: Warszawa, Poland, 1984; pp. 194–207. [Google Scholar]
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/73 (accessed on 18 December 2022).
- Boursot, P.; Auffray, J.-C.; Britton-Davidian, J.; Bonhomme, F. The Evolution of House Mice. Annu. Rev. Ecol. Evol. Syst. 1993, 24, 119–152. [Google Scholar] [CrossRef]
- Motuzko, A.; Ivanov, D. Holocene Micromammal Complexes of Belarus: A Model of Faunal Development during Interglacial Epochs. Acta Zool. Cracov. 1996, 39, 381–386. [Google Scholar]
- Bauer, K.; Spitzenberger, F. The Recent Mammal Fauna of Austria. Hystrix Ital. J. Mammal. 1996, 8, 17–21. [Google Scholar] [CrossRef]
- Cichocki, J. Mysz domowa Mus Musculus Linnaeus, 1758. In Gatunki Obce w Faunie Polski. Wyd. Internetowe; IOP PAN: Kraków, Poland, 2012. [Google Scholar]
- Kubik, J. Biomorphologische Beobachtungen Über Die Mus Musculus Linnaeus, 1758 Population Aus Einer Steinkohlengrube; Obserwacje Biomorfologiczne Nad Populacją Mus Musculus Linnaeus, 1758 z Kopalni Węgla. Acta Theriol. 1960, 4, 1–10. [Google Scholar] [CrossRef]
- Kowalski, K. Ssaki (Mammals). In Tatrzański Park Narodowy; Wydawnictwo Popularnonaukowe Zakładu Ochrony Przyrody PAN: Kraków, Poland, 1962; pp. 363–388. [Google Scholar]
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/191 (accessed on 27 November 2022).
- Pielowski, Z.; Kamieniarz, R.; Panek, M. Raport o Zwierzętach Łownych w Polsce; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 1993. [Google Scholar]
- Okarma, H. Piżmak Ondatra Zibethicus Linnaeus, 1766. In Gatunki Obce w Faunie Polski. Wyd. Internetowe; IOP PAN: Kraków, Poland, 2012. [Google Scholar]
- Brzeziński, M.; Romanowski, J.; Żmihorski, M.; Karpowicz, K. Muskrat (Ondatra Zibethicus) Decline after the Expansion of American Mink (Neovison Vison) in Poland. Eur. J. Wildl. Res. 2010, 56, 341–348. [Google Scholar] [CrossRef]
- Okarma, H.; Bartoszewicz, M.; Solarz, W. Karta Informacyjna Gatunku Piżmak Ondatra zibethicus Linnaeus, 1766; Analiza stopnia inwazyjności gatunków obcych w Polsce wraz ze wskazaniem gatunków istotnie zagrażających rodzimej florze i faunie oraz propozycją działań strategicznych w zakresie możliwości ich zwalczania oraz Analiza dróg niezamierzonego wprowadzania lub rozprzestrzeniania się inwazyjnych gatunków obcych wraz z opracowaniem planów działań dla dróg priorytetowych; GDOŚ: Warszawa, Poland, 2018.
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/64 (accessed on 18 December 2022).
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/606 (accessed on 27 November 2022).
- Cichocki, J. Szczur Śniady Rattus rattus. In Gatunki Obce w Faunie Polski. Wyd. Internetowe; IOP PAN: Kraków, Poland, 2012. [Google Scholar]
- Cichocki, J.; Ruprecht, A.L.; Ważna, A. Występowanie Szczura Śniadego (Rattus rattus L.) w Zachodniej Polsce. In Zmiany w Populacji Ssaków jako Pochodna Dynamiki Zmian Środowiska; Akademia Rolnicza im. Hugona Kołłątaja: Kraków, Poland, 2005; pp. 104–112. [Google Scholar]
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/75 (accessed on 27 November 2022).
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/607 (accessed on 27 November 2022).
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/74 (accessed on 27 November 2022).
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/200 (accessed on 27 November 2022).
- Lewartowski, Z.; Zimowski, M. Obserwacje Nutrii, Myocastor Coypus (Molina 1972) Poza Fermami Hodowlanymi. Prz. Zool. 1986, 30, 111–113. [Google Scholar]
- Nutrie Zamieszkały w Rybniku. Tu Nikt Nie Zrobi z Nich Futra! Available online: https://www.fakt.pl/wydarzenia/polska/slask/rybnik-mieszkaja-tu-nutrie-tu-nikt-nie-zrobi-z-nich-futra/mspnrqt (accessed on 27 November 2022).
- Śląskie: Martwe Nutrie w Stawie. Ktoś Bestialsko Zabił Zwierzęta? [WIDEO]. Available online: https://tvs.pl/informacje/slaskie-martwe-nutrie-w-stawie-ktos-bestialsko-zabil-zwierzeta-wideo/ (accessed on 27 November 2022).
- Chrobok, P. Rybnik. Nutrie Znad Nacyny Zniszczyły Brzeg Rzeki. Wody Polskie Musiały je Umocnić. Inaczej Mogłoby Nawet Dojść Do Podtopień. Available online: https://dziennikzachodni.pl/rybnik-nutrie-znad-nacyny-zniszczyly-brzeg-rzeki-wody-polskie-musialy-je-umocnic-inaczej-mogloby-nawet-dojsc-do-podtopien/ar/c1-16974943 (accessed on 27 November 2022).
- Mazurska, K.; Solarz, W.; Okarma, H. Karta Informacyjna Gatunku Królik Oryctolagus cuniculus Linnaeus, 1758; Analiza stopnia inwazyjności gatunków obcych w Polsce wraz ze wskazaniem gatunków istotnie zagrażających rodzimej florze i faunie oraz propozycją działań strategicznych w zakresie możliwości ich zwalczania oraz Analiza dróg niezamierzonego wprowadzania lub rozprzestrzeniania się inwazyjnych gatunków obcych wraz z opracowaniem planów działań dla dróg priorytetowych; GDOŚ: Warszawa, Poland, 2018.
- Gatunki Obce w Polsce. Available online: https://www.iop.krakow.pl/ias/gatunki/190 (accessed on 27 November 2022).
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/54 (accessed on 27 November 2022).
- Nowak, E. Rozmieszczenie, Dynamika Ilościowa i Znaczenie Dzikiego Królika, Orycłolagus Cuniculus (Linnaeus, 1758) w Polsce. Acta Theriol. 1968, 13, 75–98. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Kołodziej-Sobocińska, M.; Ruczyńska, I.; Wójcik, J.M. Range Expansion of the Golden Jackal (Canis aureus) into Poland: First Records. Mammal Res. 2015, 60, 411–414. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Wudarczyk, M.; Wójcik, J.M.; Okarma, H. Northernmost Record of Reproduction of the Expanding Golden Jackal Population. Mamm. Biol. 2020, 100, 107–111. [Google Scholar] [CrossRef]
- Atlas Ssaków Polski. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/204 (accessed on 27 November 2022).
- Mańko, O. The Proposal for Monitoring of Golden Jackal (Canis aureus). Univ. Technol. Stetin. Agric. Aliment. Pisc. 2020, 4, 31–44. [Google Scholar] [CrossRef]
- Trouwborst, A.; Krofel, M.; Linnell, J.D.C. Legal Implications of Range Expansions in a Terrestrial Carnivore: The Case of the Golden Jackal (Canis aureus) in Europe. Biodivers. Conserv. 2015, 24, 2593–2610. [Google Scholar] [CrossRef]
- Rutkowski, R.; Krofel, M.; Giannatos, G.; Ćirović, D.; Männil, P.; Volokh, A.M.; Lanszki, J.; Heltai, M.; Szabó, L.; Banea, O.C.; et al. A European Concern? Genetic Structure and Expansion of Golden Jackals (Canis aureus) in Europe and the Caucasus. PLoS ONE 2015, 10, e0141236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).