Abstract
An undescribed species belonging to the family Eunicidae was detected in a sublittoral habitat of the southern coast of Korea. This Korean eunicid species was initially thought to belong to the genus Lysidice based on its general appearance, including the absence of prostomial lateral palps and peristomial cirri. However, a more detailed characterization of the morphological features of the maxillae and mandible coupled with mtCOI and 18S rRNA gene sequence analyses confirmed that this species is a member of the genus Paucibranchia. The absence of lateral palps found in the intact adult specimens with 153 segments is a unique feature not previously reported in species belonging to Paucibranchia. Thus, the new species, Paucibranchia triantennata sp. nov., can be easily distinguished from other known congeneric species. Except for the absence of lateral palps, P. triantennata sp. nov. resembled P. conferta, P. gathofi, and P. patriciae in the shape of the prostomium, brief location and shape of branchiae, and absence of compound spinigers. However, P. triantennata could be clearly distinguished from these species by the shorter prostomial antennae, a large number of subacicular hooks, and the morphological features of the maxillae and compound falcigers.
1. Introduction
Polychaete species belonging to the family Eunicidae Berthold, 1827 [1] are found in most marine benthic environments, including Europe, America, Asia, and Africa, and inhabit the crevices of hard substrates, such as reefs, rocks, or sand [2,3]. These organisms possess well-developed jaws and are mainly carnivores or omnivores in marine benthic ecosystems [4]. Eunicid polychaetes are valued as high-quality baits for recreational and commercial fishing and are also used as a nutritional resource and maturation diet in crustacean and fish aquaculture. Therefore, these organisms are naturally caught or artificially farmed in various subtropical and temperate regions [5,6,7]. Particularly, some species belonging to this family have been subjected to large-scale aquaculture or intercontinental trade due to their commercial demand [8]. However, these polychaetes have become invasive species in some regions, causing ecological disturbances or unintended disease transmission, and have, therefore, recently garnered increasing attention [8,9].
The family Eunicidae is morphologically characterized by the presence of well-developed jaws (i.e., maxillae and mandibles), prostomial appendages, and pectinate chaetae on the parapodial lobe. This family can be distinguished from closely related families in the order Eunicida by the presence of five prostomial appendages (three antennae and two lateral palps) without annuli at the base and asymmetric maxillae (right maxilla III is missing) [10]. To date, 33 genera have been described within the family Eunicidae, 11 of which have been taxonomically validated, and the family includes approximately 460 species [2]. These 11 valid genera can be classified according to detailed features of the maxillae, prostomial appendages, peristomial cirri, branchiae, and the types of chaetae. Lysidice (Lamarck, 1818) is the only genus with fewer than three prostomial appendages, whereas all other genera have five appendages. In recent taxonomic studies, Marphysa Quatrefages, 1866 [11] and Eunice Cuvier, 1817 [12], the most representative and speciose genera in the family, have been subdivided based on detailed morphological characteristics and genetic relationships [13,14,15,16]. At the species level, M. sanguinea [17] (including its complex), a cosmopolitan species and a type species of the genus Marphysa, has been newly reported as an endemic species in Asia, America, Europe, and Africa [18,19,20]. It is also worth noting that many of these recent taxonomic studies are based on comprehensive morphological and molecular analyses and are, therefore, highly reliable.
Despite global interest in the ecology, economics, and taxonomy of eunicid polychaetes, taxonomic studies in Korea are extremely limited. There are 14 recorded species in Korea. Very limited taxonomic descriptions and drawings are available for the 11 validated species, and the absence of type specimens makes it difficult to confirm the taxonomic identity of these Korean eunicid species. Particularly, several Korean species, including Marphysa sanguinea, have been used for numerous ecological studies without taxonomic verification since they were first reported with a brief description approximately 30 years ago [21,22]. Recently, Choi et al. [23] and Kim et al. [22] reported previously unrecorded species in Korean waters based on morphological and genetic characteristics and compared these with closely related cryptic species. Nevertheless, the diversity of eunicid species in Korean waters is very low compared to the total number of species in the family, and only one new species (i.e., Leodice duplexa Choi, Kim, Kang and Yoon, 2017 [23]) has been reported to date.
Here, we identified a previously undescribed eunicid taxon in the subtidal zone of southern Korea. The individuals belonging to this newly discovered eunicid species are characterized by the presence of only three antennae on their rounded prostomium (without lateral palps), the absence of peristomial cirri, and the presence of branchiae restricted to the anterior chaetigers. These morphological features are not consistent with the diagnostic features of known Eunicidae genera. Therefore, this study aimed to confirm the taxonomic position of this Korean taxon based on comprehensive morphological and molecular analyses. In this study, we evaluated the detailed morphological features of this newly discovered Korean species, including the maxillae, parapodia, chaetae, and branchia. Additionally, molecular analyses based on the partial mitochondrial COI and nuclear 18S rRNA regions were conducted, after which these molecular signatures were compared to those of closely related taxa within the family.
2. Materials and Methods
Samples were collected from the sublittoral area near Chuja-do and Jeju-do, southern Korea, using a 0.1 m2 Smith McIntyre grab sampler (Figure 1). Sediment samples were elutriated over a 1 mm sieve using filtered seawater. The organisms remaining on the sieve were transferred to a 1 L collecting jar containing a 7% MgCl2 solution as an anesthetic agent. The relaxed specimens were fixed in a 5% buffered formalin solution within 2 h and then preserved in 80% ethanol. The eunicids were then taken to the laboratory, where they were sorted and identified to the species level under a zoom stereomicroscope (SMZ745T; Nikon, Tokyo, Japan). If necessary, the specimens were stained with Shirlastain A stain solution (SDLATLAS, Rock Hill, SC, USA) to observe their detailed morphological features. The species-specific morphological features of the examined eunicid specimens were photographed and measured using a camera (DS-Fi3; Nikon, Tokyo, Japan) attached to a stereomicroscope coupled with the NIS-Elements BR software (version: 5.11.00; Nikon, Tokyo, Japan). Given that most specimens were incomplete, the measurements were standardized by the length through the 10th chaetiger (L10) and width at the 10th chaetiger, excluding parapodia (W10). Holotype and paratypes were deposited at the Marine Biodiversity Institute of Korea (MABIK) collection in Seocheon, Republic of Korea (Table 1).
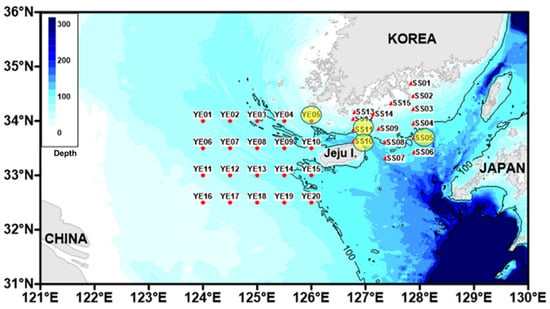
Figure 1.
Map of the study area. The red dots indicate the collection sites, and the yellow circles are the areas where samples were obtained.

Table 1.
List of species names examined in this study, as well as their GenBank accession numbers, collection sites, and associated references.
Genomic DNA was extracted from three ethanol-preserved specimens. Body tissues were partially dissected (ca. 1–2 segments) from the terminal part of the examined specimens. In order to extract the genomic DNA, 1.5 mL microcentrifuge tubes containing 45 μL of 10% Chelex suspension (Bio-Rad Laboratories Inc., Hercules, CA, USA), 10 μL of Proteinase K (10 mg/mL, iNtRON Biotechnology, Inc., Seongnam, Republic of Korea), and dissected tissues were incubated at 56 °C for 3–12 h. The extracted genomic DNA was used as a template to amplify the target regions. Polymerase chain reaction (PCR) was performed on a TaKaRa PCR Thermal Cycler Dice® Gradient (TP600; Takara Co., Kusatsu, Japan) using the polyLCO and polyHCO primer pair for mtCOI [30] and the 18A and 18B primer pair for 18S rRNA [31]. The PCR mixtures contained 17 μL of deionized water, 1 μL of each primer (10 μM), 1 μL of DNA template, and PCR premix (20 μL; BiONEER Co., Daejeon, Republic of Korea). The temperature profile for the amplification of mtCOI consisted of 94 °C/300 s; followed by 40 cycles at 94 °C/45 s, 46 °C/45 s, and 72 °C/60 s; and finally, 72 °C/420 s. For the amplification of the 18S rRNA fragment, the temperature profile consisted of an initial 94 °C/300 s step; followed by 35 cycles at 94 °C/60 s, 54 °C/60 s, and 72 °C/60 s; and finally 72 °C/420 s. The purification and sequencing of the obtained PCR products were performed at Macrogen Inc. (Seoul, Republic of Korea). Forward and reverse sequences were edited using Chromas version 2.3 (Technelysium Pty. Ltd., South Brisbane, Australia). Partial sequences of the mtCOI and 18S rRNA genes were aligned with available sequences for related taxa obtained from the GenBank database (www.ncbi.nlm.nih.gov/Genbank (accessed on 14 December 2022)) using the MEGA (Molecular Evolutionary Genetics Analysis) software (version 7.0) [32]. Table 1 provides information for all sequences used in the analyses. The aligned sequences were used to estimate genetic distances using Kimura’s two-parameter (K2P) model [33]. K2P distances were used to evaluate intraspecific variation within the Korean specimens and interspecific differences between closely related taxa. Phylogenetic relationships were inferred using the maximum likelihood method based on the General Time Reversible model [34]. Branch support was evaluated by 1000 bootstrap replicates. In this manuscript, the abbreviation MI to MV means maxillae I to V.
3. Results
3.1. Systematics
Family Eunicidae Berthold, 1827 [1].
Genus Paucibranchia Molina-Acevedo, 2018 [16].
Type species Paucibranchia bellii [35].
3.2. Generic Diagnosis
Prostomium is undivided or bilobed; three or five prostomial appendages are without articulations. Peristomium is without peristomial cirri. Maxillary apparatus have four paired maxillae plus an unpaired one (left side); MI has a developed and rounded falcal arch, with the outer edge of the base straight and with a curvature in the basal inner edge. Branchiae is restricted to a short anterior region. Dorsal cirri in the branchial region are elongated, thicker, or the same size as the basal branchial filaments. Postbranchial cirri are slender and long or longer than in pre-branchial chaetigers. Postchaetal lobes in the branchial region are well-developed and longer than in the pre-branchial region. Ventral cirri with a swollen base are only observed in the anterior region. Supracicular chaetae include limbate and two types of pectinate chaetae. Subacicular chaetae include compound falcigers, spinigers, or both. Compound chaetae have blades of different sizes in the same chaetigers or blades of only one size. Subacicular hook bidentate, or unidentate. Pygidium has two pairs of anal cirri without articulation.
3.3. Material Examined
Paucibranchia triantennata sp. nov.
urn:lsid:zoobank.org:pub:878BE10A-27B7-45B2-A9A6-0362E12FA552
The holotype was MABIKNA00157766, Republic of Korea, Chuja-do, Station SS11 (33°50′07″ N, 126°47′58″ E); subtidal zone, 84 m depth; collected by Dae Hun Kim, September 2021. The Paratypes were MABIKNA00157767, MABIKNA00157768, and MABIKNA00157769, Republic of Korea, Chuja-do, Station YE05 (34° N, 125°30′ E); subtidal zone, 75 m depth; collected by Dae Hun Kim, June 2021. Eight additional specimens were obtained from Station SS05 (33°39′45″ N, 127°53′30″ E; 109 m depth), SS10 (33°37′40″ N, 126°48′32″ E; 135 m depth), and SS11 and were used for DNA analysis and observation of morphological features including the maxillary apparatus.
3.4. Species Diagnosis
Prostomium is undivided, with only three prostomial appendages. Lateral antennae reach the first peristomial ring; median antenna reach the second peristomial ring. Branchiae pectinate has up to five filaments in chaetigers 10–18. Dorsal cirri in the post-branchial region are similar in size to the pre-branchial region cirri. Postchaetal lobes in chaetigers 2–21 are well-developed and longer than chaetal lobes. Postchaetal lobes in chaetigers 22–36 are weakly developed and similar in size to chaetal lobes. Ventral cirri with the swollen base present in chaetigers 5–39; most developed in chaetigers 10–21. Compound spinigers are absent. Compound falcigers with blades of two different sizes in the same chaetigers. Subacicular hook bidentate, starting from chaetigers 21, have one or two hooks per chaetiger.
3.5. Description
Holotype intact, has 153 chaetigers, L10 = 2.5 mm, W10 = 0.6 mm, Total length = 34.7 mm. The anterior region has a convex dorsum and flat ventrum, without groove; the body is widest at chaetiger 6, tapering after chaetiger 20.
Prostomium undivided, 0.46 mm long, 0.45 mm wide, frontally rounded, without lateral palps and median groove (Figure 2B), with ventral groove (Figure 2C). Short prostomial appendages are arranged in a semicircle, equidistant; lateral and median antennae reach the first peristomial ring. Eyes present, are rounded and brown, located on the left and right edges of the lateral antennae. Peristomium is wider than prostomium (approximately 1.27 times wider); separation between peristomial rings is distinct on all sides (Figure 2B). An inferior lip is not observed.
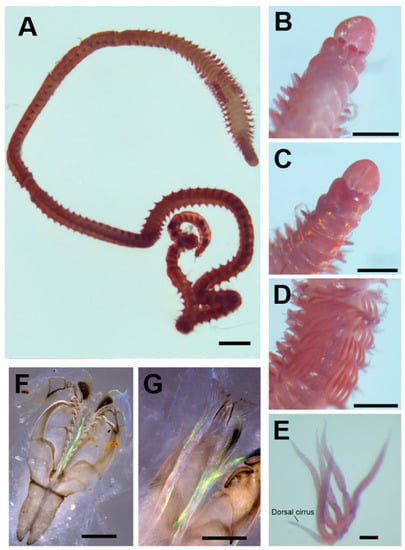
Figure 2.
Paucibranchia triantennata sp. Nov: (A) holotype, intact specimen; (B) prostomium, dorsal view; (C) prostomium, ventral view; (D) branchiae in chaetigers 12–18; (E) branchial filaments of chaetiger 15; (F) maxillae; (G) mandible. Scale bars: 1 mm (A); 500 μm (B–D); 100 μm (E–G).
Maxillary apparatus has a maxillary formula = 1 + 1, 8 + 8, 4 + 0, 3 + 5, 1 + 1 (Figure 2F). Maxillary carriers are 1.5 times shorter than MI length. MI is forceps-like. The closing system is approximately six times shorter than the MI length. The ligament between MI and MII is not sclerotized. MII is wide, the teeth are recurved, and the cavity opening is oval, approximately three times shorter than the length of MII. Short MIII has triangular teeth and attachment lamella slightly sclerotized (Figure 2F). The left MIV has a smaller basal tooth, and the right MIV has teeth of similar size. Rectangular MV has a short-rounded tooth. The mandibles and cutting plates are translucent, with approximately seven growth rings (Figure 2G).
Branchiae pectinate has up to five filaments present in chaetigers 11–17 (Figure 2A,D). The numbers of branchial filaments per chaetiger in anterior-posterior order are as follows: 4, 4, 5, 5, 5, 5, 4. The basal branchial filament is two times longer than the dorsal cirrus (Figure 2E).
The first parapodia are the smallest, most developed in chaetigers 2–21, becoming gradually smaller. Conical dorsal cirri increase in size from chaetiger 2 and gradually decrease in width and length from chaetiger 22. Dorsal cirri in the post-branchial region are similar in size to the pre-branchial region cirri. Prechaetal lobes are observed as a transverse fold in all chaetigers (Figure 3A–D). Chaetal lobes in chaetigers 1–21 are rounded and shorter than postchaetal lobes, with aciculae emerging dorsal to the midline. From chaetiger 21, lobes are triangular and longer than pre- and postchaetal lobes, with acicula emerging in the midline (Figure 3A–D). Postchaetal lobes are well developed in chaetigers 2–21; digitiform in first four chaetigers, bluntly conical from chaetiger 5 to chaetiger 21; thinner and elongated in the branchial region; decreasing in chaetigers 22–39, with following lobes being inconspicuous (Figure 3A–D). Digitiform ventral cirri are observed in chaetigers 1–4, with a swollen oval base and digitiform tip in chaetigers 5–39, most developed in chaetigers 10–21, and gradually reducing in size posteriorly from chaetiger 22 (Figure 3A–D).
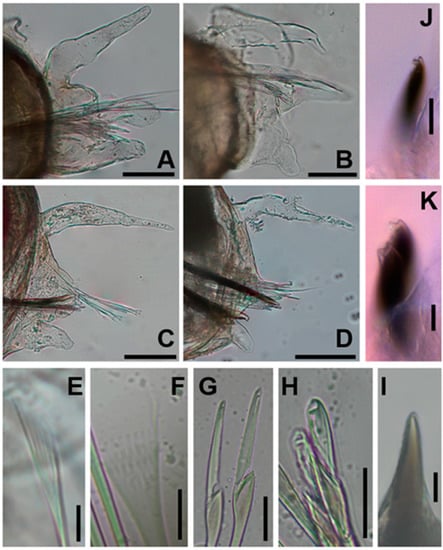
Figure 3.
Paucibranchia triantennata sp. Nov: (A) parapodium of chaetiger 4; (B) parapodium of chaetiger 15; (C) parapodium of chaetiger 25; (D) parapodium of chaetiger 58; (E) isodonts pectinate and narrow with long and slender teeth, chaetiger 3; (F) isodonts pectinate and narrow with short and slender teeth; (G) compound falciger, long blade, chaetiger 12; (H) compound falciger, short blade, chaetiger 61; (I) acicula, chaetiger 68; (J) subacicular hook, chaetiger 26; (K) subacicular hook, chaetiger 39. Scale bars: 100 μm (A–D); 20 μm (G,H,J); 10 μm (E,F,H,I,K).
Aciculae are blunt and amber-colored (Figure 3I). Only one acicula presents per chaetiger.
Limbate chaetae of two sizes are present in the same chaetiger and larger in the anterior region, reduced in number around chaetiger 22. Two types of pectinate chaetae are present. In the anterior chaetigers, isodonts are narrow with long and slender teeth, with 1–3 pectinate chaetae per chaetiger and up to 5–6 teeth with oblique distal edges (Figure 3E). Median-posterior chaetigers feature narrow isodonts with short and slender teeth, in addition to 4–5 pectinate chaetae per chaetiger and up to 8–10 teeth with oblique distal edges (Figure 3F). Compound spinigers are absent in all chaetigers. Compound falcigers are present in all chaetigers. The anterior region exhibits blades of two sizes (44 μm and 20 μm), with the smaller ones being more abundant; all teeth are triangular, and the distal tooth is directed upward and two times longer than the proximal one at longer blades. This tooth is similar in size to the proximal one at smaller blades, and the proximal tooth is directed laterally. All blades of the median-posterior chaetigers are of similar size, similar to the smaller blades of anterior chaetigers (22 μm). All teeth are triangular and similarly sized, distal tooth directed upward, whereas the proximal tooth is directed laterally (Figure 3G,H). Subacicular hooks are bidentate with a reddish basal end, distally translucent, starting from chaetigers 21, with one or two hooks per chaetiger and rounded teeth; the distal tooth is slightly smaller than the proximal tooth and directed upward, whereas the proximal tooth is directed laterally (Figure 3J,K). Pygidium has two pairs of anal cirri; the dorsal pair is as long as the last four chaetigers, and the ventral pair is short, as long as the last chaetiger.
3.6. Morphological Variation
The examined specimens varied in the following features: L10 = 2.5–2.86 mm, W10 = 0.5–0.75 mm. The maxillary formula varied as follows: MII 8–9 + 8, MIII 4–5, MIV 3–4 + 5–6. The proportion of the maxillary apparatus varied as follows: the maxillary carriers were 1.7–2 times shorter compared to the MI; the closing system was 6–7 times shorter compared to the MI; the cavity opening was 3–3.2 times shorter compared to MII. Branchiae were present from chaetigers 10–12 to chaetigers 16–18. The maximum number of branchial filaments varied from 4 to 6. Ventral cirri with swollen base were present from chaetigers 4–5 to chaetigers 37–41. Subacicular hooks began to be observed in chaetigers 21–29.
3.7. Etymology
The proposed species name triantennata refers to the unique feature of the new species, meaning three (tri) antennae (antennata).
3.8. Distribution and Ecology
Paucibranchia triantennata was sampled from three subtidal stations (water depth: 75–135 m) in southern Korea in April, June, and September 2021. The surface sediment at the station was mainly sandy mud. The salinity range at the sampling locations was approximately 31–33.9.
3.9. Molecular Comparison
In order to verify the genetic distance between the examined individuals or species, partial sequences of mitochondrial (mtCOI) and nuclear (18S rRNA) genes were used to calculate K2P distances. The intraspecific distances in mtCOI (OM158712–OM158714) and 18S rRNA (OM230034–230035) of the Korean eunicid species were very low (0–1.1% and 0%, respectively). Our mtCOI gene comparison analyses indicated that the Korean eunicid species examined in this study were genetically distinct from 18 eunicid species belonging to six genera (19.8–31.2%). Among these species, an undescribed Paucibranchia species from the Philippines (JX559753) was the most closely related (19.8%) to the newly identified species, whereas Lysidice ninetta from Portugal was the least related (31.2%). The reported genetic divergence in the mtCOI gene among polychaete species from Korean waters is 12.3–23.7%, and the interspecific genetic difference between the examined congeneric eunicid species in this study was 23.1% [22,36,37]. Therefore, the genetic difference between the Korean species and the 18 examined eunicid species was clear at the species level. Based on 18S rRNA gene sequences, the Korean species was identical to two known species of Paucibranchia (GQ497505 and AF412789) and showed a 0.4% difference from P. disjuncta (GQ497504) in the same genus. The mean genetic distances to the members of the remaining five genera within the family Eunicidae were 0.3–2.8%; for Marphysa (0.3–0.4%) and Palola Gray in Stair, 1847 [38] (0.3%) species were more closely related; and for Lysidice (0.6–1.1%) and Leodice Lamarck, 1818 [39] (1.3–2.8%) species were more distant. These genetic distances were consistent with the relationships observed in the phylogenetic trees based on the mtCOI dataset or concatenated dataset of mtCOI and 18S rRNA (Figure 4).
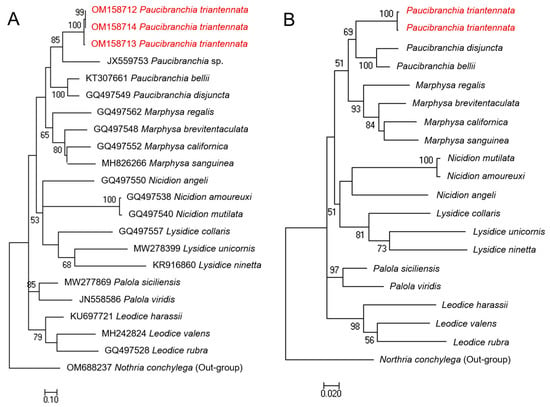
Figure 4.
Molecular phylogenetic analysis conducted via the maximum likelihood method (General Time Reversible model): (A) phylogenetic tree using mtCOI, (B) phylogenetic tree using concatenated dataset of 18S rRNA and mtCOI. The alphanumerical identifiers in front of the species name indicate the GenBank accession number. The taxon Northria conchylega, a member of the family Onuphidae, is used as an out-group in these phylogenetic trees.
3.10. Remarks
Paucibranchia triantennata sp. nov. described in this study exhibited only three antennae on the prostomium and lacked lateral palps, which distinguish it from other species belonging to this genus. Among 19 known Paucibranchia species, P. triantennata sp. nov. resembles P. conferta (Moore, 1911) [40]; P. gathofi Molina-Acevedo, 2018 [16]; and P. patriciae Molina-Acevedo, 2018 [16], particularly due to its small body size, prostomium shape (not bilobed), number of chaetigers with branchiae (5–7 chaetigers), number of branchial filaments (4–10 per branchia), relative length of dorsal cirri in post- and pre-branchial regions (similar in both regions), and absence of compound spinigers throughout the body. However, P. conferta and P. patriciae differ from P. triantennata sp. nov. in that they have one size type of blade on the compound falcigers on the anterior body (two size types in P. triantennata), longer prostomial antennae reaching up to the first and second chaetiger, and fewer subacicular hooks per parapodium. Paucibranchia gathofi and P. triantennata sp. nov. each have two blade sizes on their compound falcigers on the anterior chaetigers; however, only one size of the blade was found on the compound falcigers on the posterior chaetigers in the new Korean species. Additionally, the former is distinguished from the Korean species by having fewer subacicular hooks per parapodium (1 vs. 1–2) and a different number of branchial filaments per chaetiger (6–10 vs. 4–6).
4. Discussion
We initially assumed that the undescribed eunicid species that we retrieved from Korean waters belonged to the genus Lysidice based on its prominent morphological features, such as the absence of prostomial lateral palps and peristomial cirri. Although the Korean species of this study had branchiae only on the limited anterior chaetigers and all currently described species belonging to the genus Lysidice species do not have branchiae, the presence/absence of branchiae was considered a species-level difference, similar to the variations observed in the genus Nicidion Kinberg, 1865 [41]. However, detailed morphological features (i.e., those requiring dissection) of the Korean eunicid species with three prostomial appendages, such as the presence of a round and developed falcal arch on maxilla I and the absence of a scoop-shaped mandible, did not match those of Lysidice. This Korean species was rather similar to Paucibranchia, which has five prostomial appendages, in that the branchiae were restricted to a few anterior chaetigers, in addition to presenting well-developed dorsal cirri and a postchaetal lobe of parapodia in the branchial region, and in the detailed morphological features of maxillae and mandible. Our molecular comparison results supported this morphological similarity despite the difference in the number of prostomial appendages between the two taxa. Particularly, the Korean eunicid species were genetically similar to Paucibranchia and Marphysa but were distinct from Lysidice. Among these closely related genera, Marphysa shows a more noticeable morphological difference from Korean species because they have branchiae on most chaetigers distributed throughout the entire body. Based on the K2P distances, Korean specimens were more genetically similar to Paucibranchia than to Marphysa, and they were also assigned to the Paucibranchia clade in our phylogenetic tree.
The absence of lateral palps observed in the new Korean species can be considered a temporary feature found in a specific juvenile stage (i.e., the “Lysidice stage” bearing only the median and lateral antennae) of eunicid species. In order to confirm this, we evaluated the morphological criteria that characterize adults in the records of previously reported eunicid species. According to Herpin [42], if the total number of chaetigers exceeds 70 and the minimum length up to the 10th chaetiger (L10) exceeds 2.2 mm, the individual is considered an adult. Furthermore, the juveniles of eunicids without lateral palps exhibited only 40–60 chaetigers. In the case of M. sanguinea, a member of the genus Marphysa that is closely related to the genus Pauchibranchia, the first occurrence of prostomial lateral palps was confirmed in individuals with 60 segments, and the minimum length of an intact individual with typical morphological characteristics of an adult was 27 mm [43]. Among the 12 examined Korean specimens, the only intact specimen was a holotype specimen (with 153 chaetigers), and its total length and L10 were approximately 35 mm and 2.5 mm, respectively. Furthermore, all Korean specimens consecutively collected in April, June, and September from three different stations lacked prostomial lateral palps. Considering the length of the entire body and L10, the total number of chaetigers, and the continuity of appearance times, we confirmed that the Korean specimens were adults and that their lack of lateral palps was a unique species-level characteristic among the members of the genus Pauchibranchia. Variations in the number of prostomial appendages among congeneric eunicid species can also be found in the genus Lysidice. For instance, Lysidice unicornis [44] has two fewer prostomial appendages than other species in the genus. Despite its smaller number of prostomial appendages, L. unicornis belonged to the Lysidice clade or was most closely related to the two other Lysidice species examined in our molecular analysis (Figure 4). These genetic and morphological characteristics suggest that the newly identified Korean species belongs to the Paucibranchia genus rather than a new genus.
Key to species of Paucibranchia
- 1.
- Only compound spinigers...............................................................................2
- -
- Only compound falcigers.................................................................................5
- -
- Both compound spinigers and falcigers............................................................12
- 2(1).
- Postchaetal lobes bluntly conical or (only) conical in pre-branchial chaetigers, compound spinigers with blades of different sizes in the same chaetiger........................3
- -
- Postchaetal lobes tongue-shaped in pre-branchial chaetigers, compound spinigers with all blades of similar size in the same chaetiger...................P. kinbergi (McIntosh, 1910)
- 3(2).
- Compound spinigers with blades of two sizes, pectinate chaetae with oblique distal edge in anterior chaetigers..............................................................................4
- -
- Compound spinigers with blades of three sizes, pectinate chaetae with transverse distal edge in anterior chaetigers...................................... P. gilberti Molina-Acevedo, 2018
- 4(3).
- Eyes present, chaetal lobes rectangular in pre-branchial region, dorsal cirri in postbranchial region almost three times longer than anterior ones............................................................................................................. P. disjuncta (Hartman, 1961)
- -
- Eyes absent, chaetal lobes rounded in pre-branchial region, dorsal cirri in post-branchial region two times longer than anterior ones......................... P. cinari (Kurt-Sahin, 2014)
- 5(1).
- Subacicular hooks with reddish basal end, distally amber or translucent................6
- -
- Subacicular hooks translucent...........................................................................9
- 6(5).
- Postchaetal lobes digitiform in pre-branchial chaetigers, eyes absent, falcigers with all blades of similar size in anterior chaetigers............ P. miroi Molina-Acevedo, 2018
- -
- Postchaetal lobes bluntly conical in pre-branchial chaetigers, eyes present, falcigers with blades of two or three sizes in anterior chaetigers..................................................7
- 7(6).
- Dorsal cirri in post-branchial region 1.4 times longer than pre-branchial region, falcigers with blades of three sizes in anterior region, pectinate with transverse distal edge in anterior chaetigers......................................................P. purcellana (Willey, 1904)
- -
- Dorsal cirri in pre- and post-branchial chaetigers of similar size, falcigers with blades of two sizes in anterior chaetigers, pectinate with oblique distal edge in anterior chaetigers............................................................................................................ 8
- 8(7).
- Falcigers with blades of two sizes in posterior chaetigers, one subacicular hook per chaetiger, prostomium with lateral palps....................... P. gathofi Molina-Acevedo, 2018
- -
- Falcigers with blade of one size in posterior chaetigers, one or two subacicular hooks per chaetiger, prostomium without lateral palps...........................P. triantennata sp. nov.
- 9(5).
- Aciculae with reddish basal end, distally translucent........... P. conferta (Moore, 1911)
- -
- Aciculae translucent.................................................................................... 10
- 10(9).
- Eyes absent, dorsal cirri in postbranchial region two times longer than prebranchial region, ventral cirri with swollen base start from chaetiger 14........................................................................................................... P. gemmata (Mohammad, 1973)
- -
- Eyes present, dorsal cirri in pre- and postbranchial regions of similar size, ventral cirri with a swollen base from between chaetigers 1 and 7.............................................. 11
- 11(10).
- Postchaetal lobes basally oval, digitiform distal end, tilted dorsally; falcigers with blades of similar sizes in anterior region; pectinate with oblique distal edge in anterior chaetiger........................................................ P. patriciae Molina-Acevedo, 2018
- -
- Postchaetal lobes bluntly conical, digitiform distal end or bluntly conical; falcigers with blades of two sizes in anterior region; pectinate with transverse distal edge in anterior chaetigers; branchiae from chaetigers 14–17 with up to 10–11 filaments...........................................................................................................P. adenensis (Gravier, 1900)
- 12(1).
- Compound spinigers present in all chaetigers...P. totospinata (Lu and Fauchald, 1998)
- -
- Compound spinigers present only in anterior or median chaetigers; compound falcigers present in all the chaetigers............................................................................ 13
- 13(12).
- Subacicular hooks with reddish basal end, distally amber............................................................................................................P. andresi Molina-Acevedo, 2018
- -
- Subacicular hooks translucent or entirely amber.................................................. 14
- 14(13).
- More than four subacicular hooks per chaetiger........................................... 15
- -
- One or two subacicular hooks per chaetiger....................................................... 16
- 15(14).
- Postchaetal lobes digitiform in pre-branchial chaetigers, with 5–6 subacicular hooks per chaetiger in median region............................... P. stragulum (Grube, 1878)
- -
- Postchaetal lobes conical in pre-branchial chaetigers, with more than 10 subacicular hooks per chaetiger in median region..................... P. carrerai Molina-Acevedo, 2018
- 16(14).
- Compound falcigers present from chaetigers 14–22.... P. oculata (Treadwell, 1921)
- -
- Compound falciger present from first chaetiger..................................................17
- 17(16).
- Compound falcigers with blades of two sizes in anterior chaetigers................ 18
- -
- Compound falcigers with blades of similar size in anterior chaetigers; dorsal cirri in pre- and postbranchial region of similar length; postchaetal lobes bluntly conical in branchial region........................................................................P. fallax (Marion and Bobretzky, 1875)
- 18(17).
- Aciculae dark in anterior median region, postchaetal lobes tongue-shaped in pre-branchial chaetigers, pectinate chaetae with oblique distal edge in anterior chaetigers.................................................. P. bellii (Audouin and Milne-Edwards, 1833)
- -
- Aciculae amber in anterior median region, postchaetal lobes basally and with digitiform end in pre-branchial chaetigers, pectinate chaetae with transverse distal edge in anterior chaetigers.......................................................... P. sinensis (Monro, 1934)
Author Contributions
Conceptualization: M.-K.J. and H.Y.S.; methodology: M.-K.J. and D.H.K.; formal analysis: D.H.K. and M.-K.J.; investigation: D.H.K. and M.-K.J.; visualization: D.H.K.; resources: M.-K.J. and H.Y.S.; supervision: M.-K.J. and H.Y.S.; writing—original draft preparation: D.H.K. and M.-K.J.; writing—review and editing: M.-K.J. and H.Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Marine Biodiversity Institute of Korea (2022M00200, 2022M01100) and a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202130203).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the anonymous reviewers and the editors who made constructive and invaluable suggestions and comments. We also thank the captain and crew of the R/V Sae Dong Baek-Ho for their support at sea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Latreille, P.A.; Berthold, A.A. Latreille’s Naturliche Familien des Thierreichs; Mit Anmerkungen und Zusätzen; Landes-Industrie-Comptoirs: Weimar, Germany, 1827; p. 606. [Google Scholar] [CrossRef]
- Read, G.; Fauchald, K. (Eds.) World Polychaeta Database. 2021. Eunicidae Berthold, World Register of Marine Species. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=966 (accessed on 30 December 2021).
- Rouse, G.; Pleijel, F. Polychaetes; Oxford University Press: New York, NY, USA, 2001; p. 354. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [PubMed]
- Cole, V.J.; Chick, R.C.; Hutchings, P.A. A review of global fisheries for polychaete worms as a resource for recreational fishers: Diversity, sustainability and research needs. Rev. Fish Biol. Fish. 2018, 28, 543–565. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, B.K.; Kim, S.K.; Phoo, W.W.; Maran, B.A.V.; Kim, C.-H. Appropriate feeding for early juvenile stages of eunicid polychaete Marphysa sanguinea. Fish. Aquat. Sci. 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Watson, G.J.; Murray, J.M.; Schaefer, M.; Bonner, A. Bait worms: A valuable and important fishery with implications for fisheries and conservation management. Fish Fish. 2016, 18, 374–388. [Google Scholar] [CrossRef]
- Pombo, A.; Baptista, T.; Granada, L.; Ferreira, S.; Gonçalves, S.; Anjos, C.; Sá, E.; Chainho, P.; Da Fonseca, L.C.; E Costa, P.F.; et al. Insight into aquaculture’s potential of marine annelid worms and ecological concerns: A review. Rev. Aquac. 2018, 12, 107–121. [Google Scholar] [CrossRef]
- Vijayan, K.; Raj, V.S.; Balasubramanian, C.; Alavandi, S.; Sekhar, V.T.; Santiago, T. Polychaete worms—A vector for white spot syndrome virus (WSSV). Dis. Aquat. Org. 2005, 63, 107–111. [Google Scholar] [CrossRef]
- Zanol, J.; Carrera-Parra, L.; Steiner, T.; Amaral, A.; Wiklund, H.; Ravara, A.; Budaeva, N. The Current State of Eunicida (Annelida) Systematics and Biodiversity. Diversity 2021, 13, 74. [Google Scholar] [CrossRef]
- De Quatrefages, A. Histoire Naturelle des Annelés Marins et D’eau Douce. Annélides et Géphyriens; Volume Librarie Encyclopédique de Roret: Paris, France, 1866; p. 588. Available online: http://www.biodiversitylibrary.org/page/52110858 (accessed on 10 November 2022).
- Cuvier, G.; Dall, W.H.; Goode, G.B.; Latreille, P.A.; Laurillard, C.L.; Louvet, G.P.; Pierron, J.A.; Schaus, W.; Wood, W.W.; Congress, L.O. Le Règne Animal Distribué D’après son Organisation, Tome 2 Contenant les Reptiles, les Poissons, les Mollusques, les Annelids; Deterville: Paris, France, 1817; p. 532. [Google Scholar] [CrossRef]
- Zanol, J.; Halanych, K.; Struck, T.H.; Fauchald, K. Phylogeny of the bristle worm family Eunicidae (Eunicida, Annelida) and the phylogenetic utility of noncongruent 16S, COI and 18S in combined analyses. Mol. Phylogenetics Evol. 2010, 55, 660–676. [Google Scholar] [CrossRef]
- Zanol, J.; Halanych, K.M.; Fauchald, K. Reconciling taxonomy and phylogeny in the bristleworm family Eunicidae (polychaete, Annelida). Zool. Scr. 2013, 43, 79–100. [Google Scholar] [CrossRef]
- Molina-Acevedo, I.C.; Carrera-Parra, L.F. Revision of Marphysa de Quatrefages, 1865 and some species of Nicidion Kinberg, 1865 with the erection of a new genus (Polychaeta: Eunicidae) from the Grand Caribbean. Zootaxa 2017, 4241, 1–62. [Google Scholar] [CrossRef]
- Molina-Acevedo, I.C. Morphological revision of the Subgroup 1 Fauchald, 1970 of Marphysa de Quatrefages, 1865 (Eunicidae: Polychaeta). Zootaxa 2018, 4480, 1–125. [Google Scholar] [CrossRef] [PubMed]
- Montagu, G.I. Descriptions of several new or rare Animals, principally marine, discovered on the South Coast of Devonshire. Trans. Linn. Soc. Lond. 1813, 11, 1–26. [Google Scholar] [CrossRef]
- Hutchings, P.; Karageorgopoulos, P. Designation of a neotype of Marphysa sanguinea (Montagu, 1813) and a description of a new species of Marphysa from eastern Australia. Hydrobiologia 2003, 496, 87–94. [Google Scholar] [CrossRef]
- Lavesque, N.; Daffe, G.; Bonifácio, P.; Hutchings, P. A new species of the Marphysa sanguinea complex from French waters (Bay of Biscay, NE Atlantic) (Annelida, Eunicidae). Zookeys 2017, 716, 1–17. [Google Scholar] [CrossRef]
- Lavesque, N.; Daffe, G.; Grall, J.; Zanol, J.; Gouillieux, B.; Hutchings, P. Guess who? On the importance of using appropriate name: Case study of Marphysa sanguinea (Montagu, 1813). ZooKeys 2019, 859, 1–15. [Google Scholar] [CrossRef]
- Paik, E.I. Illustrated Encyclopedia of Fauna and Flora of Korea; Volume 31, Polychaeta; Ministry of Education: Seoul, Republic of Korea, 1989; p. 764.
- Kim, H.; Kim, K.Y.; Phoo, W.W.; Kim, C.H. The first record of the Marphysa victori (Polychaeta, Eunicida, Eunicidae) from Korea, with DNA Barcode Data. Anim. Syst. Evol. Divers. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, J.G.; Kang, D.W.; Yoon, S.M. A new species of Leodice from Korean waters (Annelida, Polychaeta, Eunicidae). ZooKeys 2017, 715, 53–67. [Google Scholar] [CrossRef][Green Version]
- Aylagas, E.; Borja, Á.; Irigoien, X.; Rodríguez-Ezpeleta, N. Benchmarking DNA metabarcoding for biodiversity-based mon-itoring and assessment. Front. Mar. Sci. 2016, 3, 96. [Google Scholar] [CrossRef]
- Struck, T.H.; Westheide, W.; Purschke, G. Progenesis in Eunicida (“Polychaeta,” Annelida)—Separate evolutionary events? Evidence from molecular data. Mol. Phylogenetics Evol. 2002, 25, 190–199. [Google Scholar] [CrossRef]
- Schulze, A.; Timm, L. Palolo and un: Distinct clades in the genus Palola (Eunicidae, Polychaeta). Mar. Biodivers. 2011, 42, 161–171. [Google Scholar] [CrossRef]
- Miralles, L.; Ardura, A.; Arias, A.; Borrell, Y.; Clusa, L.; Dopico, E.; de Rojas, A.H.; Lopez, B.; Muñoz-Colmenero, M.; Roca, A.; et al. Barcodes of marine invertebrates from north Iberian ports: Native diversity and resistance to biological invasions. Mar. Pollut. Bull. 2016, 112, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H.; Purschke, G.; Halanych, K.M. A scaleless scale worm: Molecular evidence for the phylogenetic placement of Pisione remota (Pisionidae, Annelida) Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark. Mar. Biol. Res. 2005, 1, 243–253. [Google Scholar] [CrossRef]
- Lobo, J.; Teixeira, M.A.L.; Borges, L.M.S.; Ferreira, M.S.G.; Hollatz, C.; Gomes, P.T.; Sousa, R.; Ravara, A.; Costa, M.H.; Costa, F.O. Starting a DNA barcode reference library for shallow water polychaetes from the southern European Atlantic coast. Mol. Ecol. Resour. 2015, 16, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.M.; Hardy, S.M.; Brown, T.M.; Macdonald, T.A.; Hebert, P.D.N. A Tri-Oceanic Perspective: DNA Barcoding Reveals Geographic Structure and Cryptic Diversity in Canadian Polychaetes. PLoS ONE 2011, 6, e22232. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Audouin, J.V.; Milne Edwards, H. Classification des Annélides et description de celles qui habitent les côtes de la France. Ann. Sci. Nat. 1833, 30, 411–425. [Google Scholar] [CrossRef]
- Jeong, M.-K.; Wi, J.H.; Suh, H.-L. A reassessment of Capitella species (Polychaeta: Capitellidae) from Korean coastal waters, with morphological and molecular evidence. Mar. Biodivers. 2017, 48, 1969–1978. [Google Scholar] [CrossRef]
- Jeong, M.-K.; Soh, H.Y.; Suh, H.-L. Three new species of Heteromastus (Annelida, Capitellidae) from Korean waters, with genetic evidence based on two gene markers. ZooKeys 2019, 869, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stair, J.B. An account of Palolo, a sea-worm eaten in the Navigator Islands, with a description by JE Gray. In Proceedings of the Zoological Society of London; Zoological Society of London: London, UK, 1847; Volume 15, pp. 17–18. [Google Scholar]
- Lamarck, J.B. Histoire Naturelle des Animaux Sans Vertèbres, Présentant les Caracteres Généraux et Particuliers de ces Animaux, Leur Distribution, Leurs Classes, Leurs Familles, Leurs Genres, et la Citation des Principales Espèces qui s’y Rapportent; Précédées d´une Introduction Offrant la Détermination des Caractères Essentiels de l´Animal, sa Distinction du Végétal et des Autres Corps Naturels, en fin, l´Exposition des Principes Fondamentaux de la Zoologie; Deterville & Verdiere: Paris, France, 1815; Volume 5, p. 612. [Google Scholar] [CrossRef]
- Moore, J.P. The polychætous annelids dredged by the USS “Albatross” off the coast of Southern California in 1904: III. Euphrosynidæ to Goniadidæ. Proc. Acad. Nat. Sci. Phila. 1911, 63, 234–318. Available online: http://www.biodiversityli-brary.org/bibliography/6885 (accessed on 10 November 2022).
- Kinberg, J.G.H. Annulata Nova. Öfversigt af Königlich Vetenskaps-Akademiens Förhandlingar. Stockholm 1865, 21, 559–574. Available online: http://www.biodiversitylibrary.org/bibliography/15534 (accessed on 9 November 2022).
- Herpin, R. Recherches biologiques sur la reproduction et le développement de quelques Annélides polychètes. Bull. Société Sci. Nat. L’ouest Fr. 1925, 5, 1–250. [Google Scholar]
- Prevedelli, D.; Massamba N’Siala, G.; Ansaloni, I.; Simonini, R. Life cycle of Marphysa sanguinea (Polychaeta: Eunicidae) in the Venice lagoon (Italy). Mar. Ecol. 2007, 28, 384–393. [Google Scholar] [CrossRef]
- Grube, A.E. Actinien, Echinodermen und Wurmen des Adriatischen und Mittelmeers nach Eigenen Sammlungen Beschrieben; J. H. Bon: Königsberg, Russia, 1840; p. 92. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).