Abstract
Thin filamentous cyanobacteria isolated from three collection sites in the Republic of Korea were suggested as three new species belonging to the genus Plectolyngbya, mainly according to their molecular characteristics. The species of Plectolyngbya, including the type species of P. hodgsonii, were cryptic species that were difficult to distinguish morphologically from each other, and had appeared in ecologically diverse habitats. P. terrestris and P. koreana were subaerophytes collected from certain black spots and soils between stone walls in Seoul, Republic of Korea. In addition, hypersaline species collected from a saltern, P. salina, shared the same halophytic feature as the P. hodgsonii from the littoral zone of a coastal lake in the Antarctic. The 16S rRNA gene phylogeny supported the monophyly of Plectolyngbya with solid support, 99% Maximum Likelihood, 98% Neighbor-Joining bootstrap support values, and 1.0 Bayesian posterior probability. The ITS sequences of P. terrestris, P. koreana, and P. salina were unique in length and nucleotide composition, with different secondary structures of D1–D1ʹ and Box-B helices, compared with those of P. hodgsonii. These results demonstrate that the proposed new Plectolyngbya species were unique in their molecular traits. Therefore, we suggest them as new species belonging to the genus Plectolyngbya with the names P. terrestris sp. nov., P. koreana sp. nov., and P. salina sp. nov.
1. Introduction
Prokaryotic cyanobacteria have an enormous morphological and ecological diversity. They are distributed in most habitats in the world, from soils to aerial habitats, including freshwater, brackish and seawater, and even extreme environments, such as ice-associated sites of polar regions, subaerial soil, and hypersaline locations [1]. Hypersaline habitats are characterized as having a high salinity, high alkalinity, low oxygen concentration, and having halophilic microorganisms [2]. Halophilic cyanobacteria are known to be one of the major bacteria in high-salt concentration environments, such as hypersaline lakes, saline springs and soils, coastal hypersaline lagoons, and saltern evaporation ponds worldwide [2,3]. The halophilic cyanobacteria, a unicellular and filamentous species, form dense benthic mats in shallow solar salterns worldwide. These halophilic cyanobacteria species have been reported [4,5] as Aphanothece by Nägeli [6]; Oscillatoria by Vaucher ex Gomont [7]; Leptolyngbya by Anagnostidis and Komárek [8]; Plectolyngbya by Taton, Wilmotte, Smarda, Elster, and Komárek [9]; and Halospirulina by Nübel Garcia-Pichel and Muyzer [10].
Cyanobacteria also inhabit subaerial biofilms formed on the surface of soils and rocks, which are one of the most extreme environments. They mostly synthesize the exopolysaccharide matrix because of their continuous exposure to the atmosphere [11,12]. The subaerophytes, Aphanothece, Leptolyngbya, Nostoc by Vaucher ex Bornet and Flahault [13]; Oculatella by Zammit, Billi, and Albertano [14]; Scytonema by Agardh ex Bornet and Flahault [15]; Tolypothrix by Kützing ex Bornet and Flahault [15]; and Westiellopsis by Janet [16] were isolated from stone temples, build facades, ancient hypogea arid deserts, and semi-arid soils.
Cyanobacteria have traditionally been identified using morphological features, although this practice has been shown to be potentially problematic due to phenotypic or culture-induced plasticity [17,18]. The taxonomic system of cyanobacteria was radically changed, particularly with the introduction of electron microscopy, as well as molecular and genetic methods for the characterization of cyanobacterial taxa [19].
Cyanobacteria currently contain 12 orders including the Synechococcales supported by molecular sequence data [20]. Among the over 5200 cyanobacterian species, a total of 34 genera and 232 species were described as Leptolyngbyaceae, Synechococcales [20]. Despite taxonomic difficulties of having a simple morphology, numerous leptolyngbyacean novel genera and species have been reported through ultrastructure and 16S rRNA and 16S–23S ITS sequences [21]. The morphotypes of Leptolyngbya (having simple and fine trichomes) were very difficult to distinguish from other sister taxa [22], i.e., Lyngbya by Agardh ex Gomont [7], Phormidium by Kützing ex Gomont [7], and Plectonema by Thuret ex Gomont [7]. The Leptolyngbya morphotypes have been identified and described as numerous novel genera, such as Alkalinema by Vaz et al. [23]; Amazoninema by Genuário et al. [24]; Nodosilinea by Perkerson and Casamatta [25]; Oculatella and Onodrimia by Jahodárová, Dvorák, and Hašler [26]; Pantanalinema by Vaz et al. [23]; Phormidesmis by Turicchia, Ventura, Komárková, and Komárek [27]; Plectolyngbya and Scytolyngbya by Song and Li [28]; Thermoleptolyngbya by Sciuto and Moro [29]; Timaviella Sciuto and Moro [30]; Toxifilum Zimba, Huang, Foley, and Linton [31].
The Plectolyngbya, belonging to the family Leptolyngbyaceae, has filamentous and false branched trichomes [21]. They are only found in periphyton and metaphyton in mats among other cyanobacteria in the littoral of Larsemann Hills’ lakes in the Antartica, and more strains were found from Antartic lakes [9]. In the strains of Plectolyngbya, the trichome morphology is most similar to Leptolyngbya, a type of false branching similar to Pseudophormidium, and the occasional multiple arrangement of trichomes in the sheaths is similar to Schizothrix by Kützing ex Gomont [7,32,33]. However, they are distinctly separated from other genera in phylogeny using 16S rRNA gene sequences, and thus a new genus, Plectolyngbya, has been described by combining molecular characterization with phenotypic and/or cytological and ecological markers [9].
In the present study, we collected and identified new leptolyngbyacean cyanobacteria from subaerial and saltern habitats of the Republic of Korea. Therefore, we would like to suggest that these new cyanobacteria are three new species belonging to Plectolyngbya, based on morphological traits observed under light microscopy (LM) and transmission electron microscopy (TEM), as well as ecological traits and molecular data (such as 16S rRNA and 16S–23S rRNA ITS).
2. Materials and Methods
2.1. Strain Isolation and Culture Conditions
Three new cyanobacterial samples were collected from three collection sites, namely two subaerial habitats (37°33′52.4″ N 126°57′43.5″ E, 37°35′58.0″ N 126°57′25.8″ E) in Seoul, Republic of Korea, and one saltern habitat (35°35′44.8″ N 126°36′44.1″ E) in Buan, Republic of Korea, respectively (Figure 1). The field samples were kept in 50 mL conical tubes (SPL Life Science, Pocheon, Republic of Korea), antisepticised distilled water, and were sealed up at the sites. After that, the samples were placed in an icebox at 4 °C [34] and transported to the laboratory of Kyonggi University, Suwon, Republic of Korea.
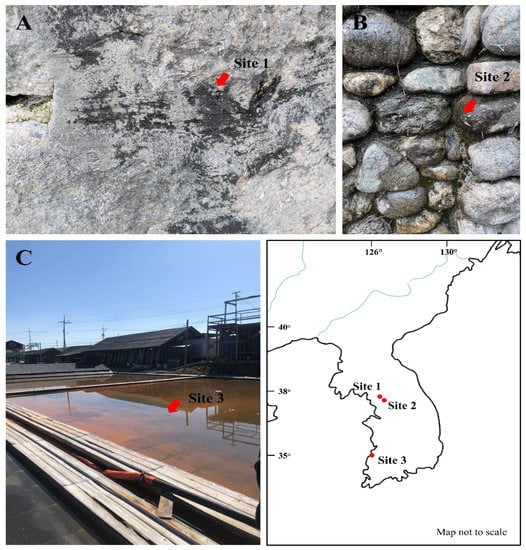
Figure 1.
Photographs showing the collection sites of (A) Plectolyngbya koreana, (B) Plectolyngbya terrestris, and (C) Plectolyngbya salina. (A) Jongno-gu, Seoul, Republic of Korea (37°35′58.0″ N 126°57′25.8″ E), (B) Seodaemun-gu, Seoul, Republic of Korea (37°33′52.4″ N 126°57′43.5″ E), (C) Buan-gun, Jeollabuk-do, Republic of Korea (35°35′44.8″ N 126°36′44.1″ E), and the habitat views of the collection sites (arrows).
For culture strain development, a single trichome was separated and washed using the Pasteur pipette (Hilgenberg GmbH, Mansfeld, Germany) and the sterile distilled water (S.D.W.) under the optical microscope. Each trichome was kept in S.D.W. for a week. In order to minimize contamination, the best clean trichome was transferred to the 50 mL cell culture flask (SPL Life Science, Pocheon, Republic of Korea) with BG-11 Medium (Sigma-Aldrich, St. Louis, MO, USA), as described in a previous study [35]. The indoor culture was carried out under the condition of light:dark for 16 h:8 h with 25 μmol·m−2 s−1 for the intensity of illumination, and 25 ± 1 °C in temperature.
The living strains were deposited to the Freshwater Bioresources Culture Collection (FBCC; https://fbp.nnibr.re.kr/fbcc/; accessed on 28 April 2022) at the Nakdonggang National Institute of Biological Resources (NNIBR) of the Republic of Korea with the following accession numbers: Plectolyngbya salina FBCC-A1477, Plectolyngbya koreana FBCC-A1478, and Plectolyngbya terrestris FBCC-A1479. The inoculated subculture of the strains was preserved in 4% (v/v) formaldehyde and was deposited in the Herbarium at the National Institute of Biological Resources (KB; https://species.nibr.go.kr/; accessed on 27 August 2021) (accession numbers NIBRCY0000001294: FBCC-A1477, NIBRCY0000001293: FBCC-A1478, and NIBRCY0000001292: FBCC-A1479).
2.2. Morphological and Ultrastructure Analyses
All of the strains were observed at a magnification of 100 to 1000 times under an optical microscope, the Olympus BX53 (Olympus, Tokyo, Japan), and pictures were taken accordingly with a digital camera, namely the Olympus UC-90 (Olympus, Tokyo, Japan).
The TEM samples were fixed with 2% glutaraldehyde and 2% paraformaldehyde in a phosphate buffer (pH 7.4) at 4 °C for 1 h, and were then postfixed with 2% osmium tetroxide and 3% potassium hexacyanoferrate at 4 °C for 40 min. The samples were dehydrated in a graded series of ethanol and embedded into LR white resin. The sections were then ultra-thin sectioned into 80 nm by using the Ultracut microtome (Leica Co., Greenwood Village, CO, USA) and were placed on a copper grid. The final samples were stained with uranyl acetate and lead citrate. The stained samples were observed using a transmission electron microscope, JEM-2100F (Jeol, Tokyo, Japan), at the Korean Basic Science Institute, Chuncheon, Republic of Korea, as described by Kim et al. [36].
The taxonomic classification system of the cyanobacteria in this study applied Komárek et al. [21] and referenced Taton et al. [9] and AlgaeBase [20].
2.3. Molecular Methods
A total of 20–25 mL of the culture and medium was harvested during the exponential growth phase and concentrated (ca. 1 g of wet weight) by centrifugation. All of the DNA were extracted using the i-genomic Plant DNA Extraction Mini Kit (iNtRON Bio, Daejeon, Republic of Korea), according to the manufacturer’s protocol.
The 16S rRNA and ITS region were amplified using the universal bacterial primers, i.e., 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and CY-23R600 (5′-CGGCTCATTCTTCAACAGGCAC-3′) [35]. The PCR reactions were done in total 20 μL volumes containing 17 μL of S.D.W., 1 μL of forward and reverse primers (10 pmoles), and with Maxime™ PCR PreMix Kit (i-StarTaq™ GH) (iNtRON Bio, Daejeon, Republic of Korea). PCR was done on the Mastercycler gradient (Eppendorf, Hamburg, Germany) with an initial denaturation at 94 °C for 5 min, followed by 35 main cycles of denaturation at 94 °C for 20 s, annealing at 55 °C for 30 s, and extension at 72 °C for 90 s, and a final extension at 72 °C for 10 min [35].
The PCR products were purified using the MEGAquick-spinTM Plus DNA Fragment Purification Kit (iNtRON Bio, Daejeon, Republic of Korea) according to the manufacturer’s protocol, and were then sequenced commercially (Macrogen Inc., Seoul, Republic of Korea). Both the forward and reverse electropherograms of each sample were trimmed and assembled using the program SeqMan (DNASTAR, Madison, WI, USA). All newly determined sequences were deposited in GenBank, accession no. OK382086–OK382088.
2.4. Phylogenetic Analysis
The 16S rRNA alignment included 52 cyanobacteria taxa. Two Gloeobacterales and three Pseudanabaenaceae were selected as the outgroup, i.e., Gloeobacter violaceus by Rippka, Waterbury, and Cohen-Bazire [37], PCC 7421 (NR_074282, 1485 bp); G. kilaueensis by Saw et al. [38], JS1 (NR_121745, 1485 bp); Pseudanabaena catenata by Lauterborn [39], SAG 254.80 (KM020004, 1458 bp); Pseudanabaena sp. PCC 6802 (AB039016, 1409 bp); and Pseudanabaena sp. PCC 7367 (AF091108, 1436 bp). The published 16S rRNA of the representative Leptolyngbyaceae [29,40] (total of 48 taxa, minimum 991 bp, average 1342 bp, and maximum 1489 bp) were obtained from GenBank and aligned using the ClustalW implemented in Geneious Prime ver. 2022.1.1 (http://www.geneious.com; accessed on 1 September 2021, Biomatters, Auckland, New Zealand), and followed by manual curation. Ambiguous positions were excluded in the subsequent analyses, in order to reduce the phylogenetic uncertainty. The 16S rRNA positions (reference sequence Plectonema sp. SAG 38.60, KM019916) that were used in the analysis were positions 1–43, 59–144, 151–398, 403–1372, and 1379–1466. Complete (total 1558 bp) and unambiguous (1435 bp) alignments are available from the authors upon request.
The best phylogeny and support for monophyletic nodes were inferred under the Maximum Likelihood (ML) method using RAxML ver. 8.2.12 [41], the Neighbor-Joining (NJ) method using MEGA X [42], and the Bayesian Inference (BI) using MrBayes ver. 3.2.7a [43]. A general time reversible model with rate heterogeneity (GTR + G) was applied for the ML and BI analyses. The ‘f-a’ option was used in RAxML for simultaneous best phylogeny tree search with rapid bootstrap analysis with ‘-# 1000′ (1000 bootstrap replications; MLB), default ‘-I’ (automatically optimized SPR branch rearrangement for heuristic search), and ‘-c’ (25 distinct rate categories). The default p-distance setting for sequence differences was used for the NJ tree search and 1000 bootstrap replications (NJB). Genetic distances among the taxa were calculated in the program MEGA X. Bayesian phylogeny and posterior probabilities inferred from 20 million generations of the Metropolis-coupled Markov Chain Monte Carlo (MC3) with default parameters, as follows: two independent runs with different random start points, one cold chain and three heated chains for each run, and tree sampling at every 100th generation. The burn-in point of chain was identified by the average standard deviation of the split frequencies (<0.01) between runs, i.e., 230,000 generations. Thus, the first 25% of generations were discarded for the Bayesian posterior probability (BPP) calculation.
2.5. Structure Analyses of 16S–23S Internal Transcribed Spacer (ITS)
Putative secondary structures of 16S–23S rRNA ITS were constructed using Mfold ver. 3.2 web-based software (http://www.unafold.org/mfold/applications/rna-folding-form.php; accessed on 1 September 2021) according to [44], and were re-drawn in PseudoViewer3 (http://pseudoviewer.inha.ac.kr/; accessed on 1 September 2021) [45] for easy comparison with the available structures of the relative taxa.
3. Results
3.1. Taxonomic Treatment
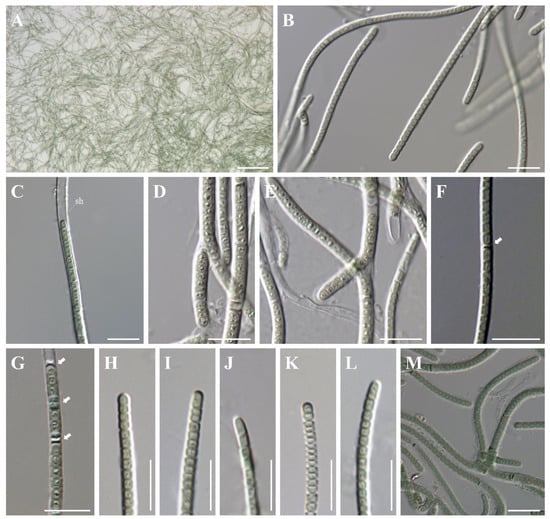
Figure 2.
Microphotographs of Plectolyngbya terrestris from the strain FBCC-A1479. (A) Colonies (mats) formed in the culture, (B,C) Detail of trichomes, (sh) sheaths, (D) Tolypothrix-type of false branching, (E) Scytonema-type of false branching, (F,G) Arrows indicate the necridic cells, (H–L) Morphologies of apical cells, (M) Trichome with sheaths after 4 weeks of culture. Scale bars (A) 100 μm, (B–M) 10 μm.
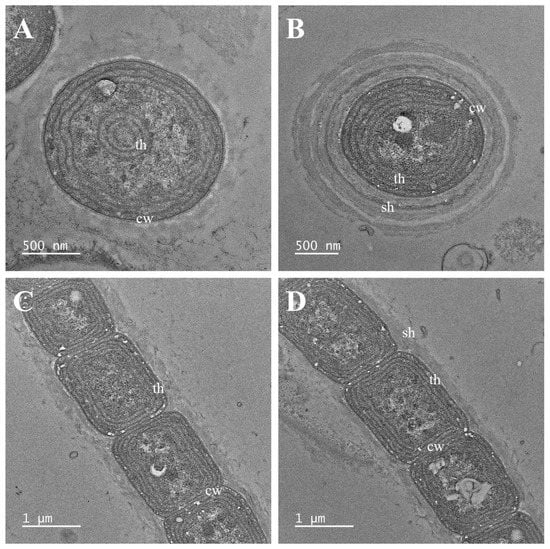
Figure 3.
Transmission electron micrographs of Plectolyngbya terrestris strain FBCC-A1479. (A,B) Parietal thylakoids seen in the cross section, (C,D) Parietal thylakoids appearing in the longitudinal section. cw: cell wall; sh: sheath; th: thylakoids.
Description: Filament solitary or in a small colony, straight or curved. Trichomes pale greyish or blue-green, not attenuated towards the ends. Cells cylindrical or slightly barrel-shaped, 1.0–2.5 μm long and 1.5–2.5 μm wide, longer or shorter than wide, clearly constricted at cross-walls, but old cells not constricted at cross-walls. Apical cell conical or rounded without calyptra. Sheaths very thin, colorless, attached from trichomes. False branching facultative in old trichome, branches solitary or in pairs. Reproduction hormogonia with necridic cells. Thylakoids parietal or circular.
Notes: Plectolyngbya terrestris is distinguished from other Plectolyngbya species by the appearance of false branching in old trichomes. Although they are most ecologically similar to P. koreana appearing in soil, they are distinguished by the difference in the total length of the D1–D1’ helix and the terminal stem length of the Box-B helix. TEM analyses also found multilayered sheaths of trichomes (Figure 3B).
Etymology: terrestris, meaning to live on soil (terra).
Holotype (designated here): A formaldehyde fixed specimen, NIBRCY0000001292 in the Herbarium at the National Institute of Biological Resources (KB), from the cultured strain FBCC-A1479.
Reference strain: FBCC-A1479.
Type locality: Seodaemun-gu, Seoul, Republic of Korea (37°33′52.4″ N 126°57′43.5″ E).
Habitat: This species is a sub-aerophytic living in the soil between rocks of the stone wall.
Gene sequences: The 16S rRNA to 23S rRNA gene sequences: GenBank accession No. OK382086.

Figure 4.
Microphotographs of Plectolyngbya koreana from the strain FBCC-A1478. (A) Colonies (mats) formed in culture, (B) Detail of trichomes, (C,D) Tolypothrix-type of false branching. (E,F) Scytonema-type of false branching, (G) Trichome with sheath, (H) Trichome with the necridic cell (nc) coming out of the sheath, (I–L) Morphologies of apical cells, (M) Trichome with sheaths (arrow) after 4 weeks of culture. Scale bars (A) 100 μm, (B–M) 10 μm.
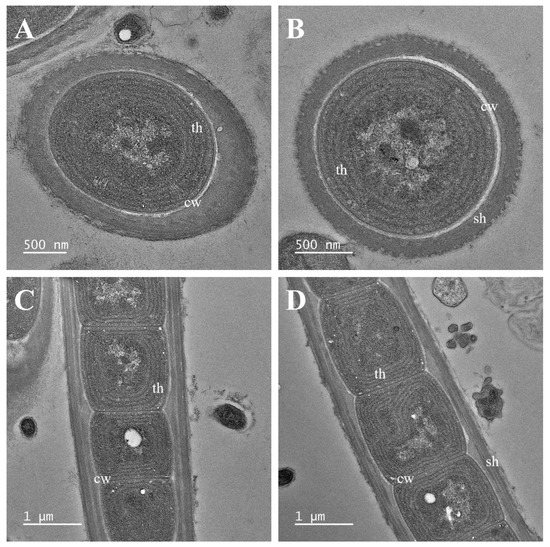
Figure 5.
Transmission electron micrographs of Plectolyngbya koreana strain FBCC-A1478. (A,B) Parietal thylakoids seen in cross section, (C,D) Parietal thylakoids appearing in the longitudinal section. cw: cell wall; sh: sheath; th: thylakoids.
Description: Filament solitary or in a small colony, straight or curved. Trichomes blue-green, not attenuated towards the ends. Cells cylindrical or slightly barrel-shaped, 1.5–3.0 μm long and 1.5–3.0 μm wide, longer or shorter than wide, clearly constricted at the cross-walls. Apical cell conical or rounded and rarely circled without calyptra. Sheaths very thin, colorless, attached or slightly widened from the trichomes, diffluent. False branching obligatory, branches solitary or in pairs. Reproduction hormogonia with necridic cells. Thylakoids parietal or circular.
Notes: Plectolyngbya koreana has slightly longer cells than the other species of Plectolyngbya. The D1–D1′ helix shows a similar loop to other species; however, the Box-B helix is distinguished by its shorter length than the other Plectolyngbya species. TEM analyses have also found multilayered sheaths of trichomes (Figure 5A–D).
Etymology: koreana, “Korea” means Korea, a collection site.
Holotype (designated here): A formaldehyde fixed specimen, NIBRCY0000001293 in the Herbarium at the National Institute of Biological Resources (KB), from the cultured strain FBCC-A1478.
Reference strain: FBCC-A1478.
Type locality: Jongno-gu, Seoul, Republic of Korea (37°35′58.0″ N 126°57′25.8″ E).
Habitat: This species is a sub-aerophytic living in the soil between rocks of the stone wall.
Gene sequences: The 16S rRNA to 23S rRNA gene sequences: GenBank accession No. OK382087.

Figure 6.
Microphotographs of Plectolyngbya salina from the strain FBCC-A1477. (A) Colonies (mats) formed in culture, (B) Trichomes twisted into a rope, (C) Details of trichomes, (D) Tolypothrix-type of false branching, (E) Scytonema-type of false branching, (F) Trichome with necridic cell (nc), (G–I) Morphologies of apical cells, (J) Trichome coming out of the sheath, (K) Trichome with slightly widened sheath (arrow) after 4 weeks of culture. Scale bars (A) 100 μm, (B–K) 10 μm.
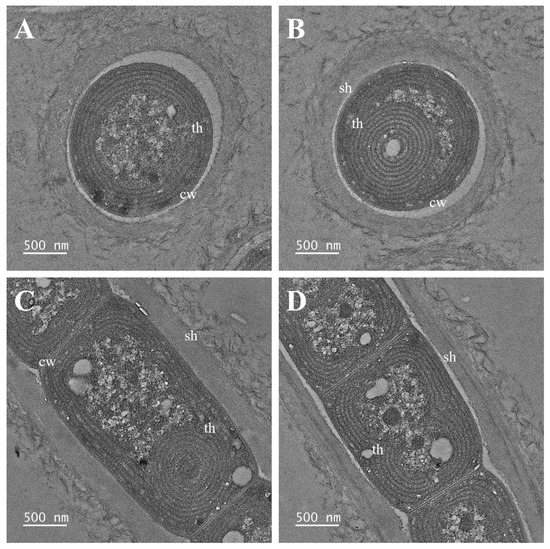
Figure 7.
Transmission electron micrographs of Plectolyngbya salina strain FBCC-A1477. (A,B) Parietal thylakoids seen in the cross section, (C,D) Parietal thylakoids appearing in the longitudinal section. cw: cell wall; sh: sheath; th: thylakoids.
Description: Filament solitary or in a small colony, straight or waved and sometimes gently twisted. Trichomes blue-green, not attenuated towards the ends. Cells cylindrical or slightly barrel-shaped, 1.0–2.5 μm long and 1.5–2.0 μm wide, longer or shorter than wide, clearly constricted at the cross-walls. Sheaths very thin, colorless, attached or slightly widened from the trichomes, diffluent. Apical cell conical or rounded without calyptra. False branching obligatory, branches solitary or in pairs. Reproduction hormogonia with necridic cells. Thylakoids parietal or circular.
Diagnosis: Plectolyngbya salina inhabit high salinity areas, which is ecologically very different from other species of Plectolyngbya. It is also molecularly distinguished from P. terrestris and P. koreana by the secondary structure of Box-B.
Etymology: salina, live in the hypersaline water in Gomso saltern, Republic of Korea.
Holotype (designated here): A formaldehyde fixed specimen, NIBRCY0000001294 in the Herbarium at the National Institute of Biological Resources (KB), from the cultured strain FBCC-A1477.
Reference strain: FBCC-A1477.
Type locality: Buan-gun, Jeollabuk-do, Republic of Korea (35°35′44.8″ N 126°36′44.1″ E).
Habitat: This species is a periphyton living in saltern water in a hypersaline state.
Gene sequences: The 16S rRNA to 23S rRNA gene sequences: GenBank accession No. OK382088.
3.2. Characteristic of 16S rRNA and Phylogeny
The newly determined 16S rRNA sequences of the tree Plectolyngbya were 1489 bp for P. terrestris FBCC-A1479 (53.6% GC content), 1483 bp for P. koreana FBCC-A1478 (53.8% GC content), and P. salina FBCC-A1477 (54.5% GC content). Unambiguous 16S rRNA alignment of 51 Leptolyngbyaceae taxa and two outgroups were 1435 sites with an overall 54.6% GC content (A: 25.1%, C: 23.0%, G: 31.6%, and T: 20.2%). The alignment contained 1003 constant sites (69.9%) and 346 parsimony-informative sites (24.1%) among the 432 variable sites (30.1%). The 16S rRNA p-distance within Leptolyngbyaceae was 7.74% (±2.8%, standard deviation) on average, and ranged from 0.14% between Leptolyngbya foveolarum (Gomont) by Anagnostidis and Komárek [8] 1964/112 (X84808) and Leptolyngbya boryana (Gomont) by Anagnostidis and Komárek [8] IAMM 101 (AB245143) to 12.88% between Leptothoe kymatousa by Konstantinou and Gkelis [46] TAU-MAC 1215 (KY744812) and Planktolyngbya limnetica (Lemmermann) by Komárková-Legnerová and Cronberg [47] PMC271.06 (GQ859645). The p-distance within Plectolyngbya 16S rRNA was 2.48% (±0.7%) on average and ranged from 1.22% (minimum; 14 nucleotide differences) between Plectolyngbya sp. WJT66-NPBG17 (KJ939022) and P. koreana FBCC-A1477 (OK382087) to 3.90% (maximum; 51 nucleotide differences) between Plectolyngbya sp. SAG 38.90 (KM019916) and P. hodgsonii ANT.LG2.1. (AY493615).
The ML phylogeny of the 16S rRNA supported the monophyly of Leptolyngbyaceae with 100% MLB, 72% NJB, and 1.00 BPP (Figure 8). The resolved inter-genus level relationship within the class was the monophyly of Cymatolege by Konstantinou and Gkelis [48], Euryhalinema by Chakraborty and Mukherjee [49], Leptothoe by Konstantinou and Gkelis [46], and Rhodoploca by Konstantinou and Gkelis [48] (98% MLB, 95% NJB and 1.00 BPP), and the monophyly of Myxacorys by Pietrasiak and Johansen [50], and Leptolyngbya sensu stricto and Plectolyngbya (94% MLB, 84 NJB, and 1.00 BPP). A sister relationship between Plectolyngbya and Leptolyngbya sensu stricto was supported by the maximum support vales of MLB, NJB, and BPP. Three new Plectolyngbya species showed independent phylogenetic positions within the monophyletic Plectolyngbya (99% MLB, 98% NJB, and 1.00 BPP). First, P. terrestris FBCC-A1479 showed a sister relationship with Plectonema sp. SAG 38.90 (KM019916) (81% MLB and 1.00 BPP), with 1.39% p-distance (20 nucleotide differences; 98.6% similarity). P. koreana FBCC-A1478 showed a closer relationship with Plectolyngbya sp. WJT66-NPBG17 (KJ939022) (62% MLB, 69% NJB, and 0.76 BPP), with a 1.22% p-distance (14 nucleotide differences; 98.78% similarity), followed by Plectonema sp. SGA 38.90 (KM019916) with a 1.5% p-distance (19 nucleotide differences; 98.5% similarity). Lastly, P. salina FBCC-A1477 showed a sister relationship with P. hodgsonii ANT.LPR2.2 (AY493583) (95% MLB, 90% NJB, and 1.00 BPP), with 1.88% p-distance (27 nucleotide differences; 98.12% similarity) (Table 1).
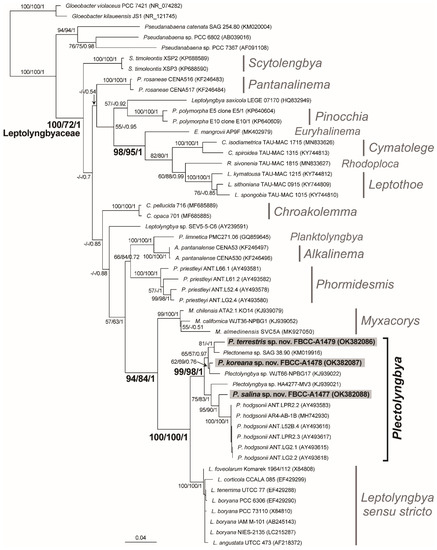
Figure 8.
Maximum likelihood (ML) phylogeny of the Plectolyngbya based on 16S rRNA sequences (1435 positions). The best tree of Leptolyngbyaceae (Synechococcales; total 47 strains 14 genus) inferred using RAxML with the GTR + G model. Two Gloeobacter (Gloeobacterales) and three Pseudanabaena (Pseudanabaenaceae, Synechococcales) were selected as the outgroups. The species name followed by strain ID and GenBank accession number within the parentheses. Leptolyngbya sensu stricto adapted from Taton et al. [9]. The ML bootstrap support (MLB), Neighbor-Joining bootstrap support (NJB), and the Bayesian posterior probability (BPP) are given near the monophyletic node, respectively.

Table 1.
The similarity (upper diagonal, %) and absolute difference (lower diagonal, base pairs) matrix of 16S rRNA among the Plectolyngbya and Leptolyngbya taxa.
3.3. Characterization of 16S–23S ITS Regions
The ITS regions of the Plectolyngbya ranged from 477 bp in P. koreana FBCC-A1478 (OK382087) to 497 bp in P. terrestris FBCC-A1479 (OK382086), including conserved domains D1–D5, tRNA-Ala, tRNA-Ile, variable stem V3, and antiterminators Box-A and Box-B (Supplementary Figure S1). The secondary structures of the D1–D1’ and Box-B helices of three new Plectolyngbya species were reconstructed (Figure 9). The total length of the D1–D1’ helices were 53–56 bp, and its basal part consisted of a 5-bp stem (5′-GACCU-AGGUC-3′), followed by a 6-bp 3′ side unilateral bulge in P. terrestris FBCC-A1479 and P. koreana FBCC-A1478 (Figure 9A,B). However, P. salina FBCC-A1477 showed a 5-bp 3′ side unilateral bulge (Figure 9C). After the 10–11 bp basal structure, P. terrestris, P. koreana, and P. salina showed different sub-helix structures. P. terrestris showed two mid-internal loops located at 9/41–42 and at 15–17/34–35, P. koreana showed two mid-internal loops located at 9/39–40 and at 17/32, and P. salina showed one mid-internal loop located at 9/39–40. The terminal loops were 6-bp of 5′-UUUGAU-3′ in P. terrestris, 4-bp of 5′-UUAG-3′ in P. koreana, and 4-bp 5′-UUCG-3′ in P. salina.
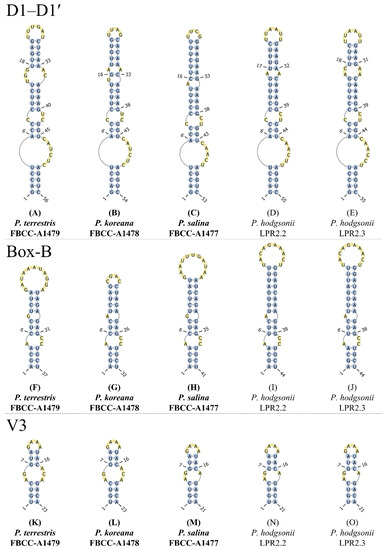
Figure 9.
Secondary structures for the D1–D1’, Box-B, and V3 in conserved regions of the 16S–23S ITS. (A,F,K) Plectolyngbya terrestris, (B,G,L) P. koreana, (C,H,M) P. Salina, and (D,E,I,J,N,O) P. hodgsonii.
The Box-B helices of all of the Plectolyngbya showed different structures (Figure 9F–J). The total lengths of the Box-B helix were 32–44 bp and all of the basal stems consisted of 4-bp (5′-AGCA-UGCU-3′). P. terrestris FBCC-A1479, P. koreana FBCC-A1478, and P. salina FBCC-A1477 showed different structures in the terminal stem and terminal loop in the Box-B helix (Figure 9F–H). The terminal stem and loops of P. terrestris FBCC-A1479 were 5′-UCUA-UAGA-3′ (at 10–13/25–28) and 5′-GAUAAAUAGGA-3′ (at 14–27), respectively (at 10/28). Those of P. koreana FBCC-A1478 were 5′-UCUAG-CUAGA-3′ (at 10–14/19–23) and 5′-CGAC-3′ (at 15–18) 10/23, and P. salina FBCC-A1477 were 5′-UCUGAU-AUCAGA-3′ (at 10–15/27–32) and 5′-GAAUUUGAUAA-3′ (at 16–26) 10/32.
The V3 helices of the three Plectolyngbya species were similar to each other in the number of stems and loops, and the total lengths were 21–23 bp, and all of them consisted of a 4-bp identical 4-bp basal stem (5′-UGUC-GACA-3′) (Figure 9K–O). The V3 helix structures of all Planktolyngbya species showed two types. For example, P. terrestris FBCC-A1479 and P. koreana FBCC-A1478 showed loops located at 5–6/17–19. However, P. salina FBCC-A1477, P. hodgsonii LPR2.2, and P. hodgsonii LPR2.3 showed loops located at 5–6/17.
4. Discussion
The taxonomic research on novel cyanobacteria species is rapidly growing after the introduction of molecular methods [21,51,52]. As the largest family in the Synechococcales, Leptolyngbyaceae have received significant attention in recent years [53]. The taxa within the Leptolyngbyaceae have been repeatedly shown to be highly divergent in their 16S rRNA sequence, which led to their being split into several new families. In particular, Leptolyngbya was classified into more than 21 genera by using 16S rRNA and habitats [53]. In the present study, three new Plectolyngbya species showed indistinguishable morphologies among each other and similarities to the type of species of P. hodgsonii. The three new species belonging to Plectolyngbya that are newly proposed in this study are also cryptic species that are morphologically indistinguishable from each other [19].
The morphology of Plectolyngbya was similar to Leptolyngbya, such as having thin and ensheathed trichomes, false branching, cell shape, and thylakoid structure [19]. In addition, multilayered sheaths of trichomes were found in the TEM analyses of P. terrestris and P. koreana. This feature was also found in the TEM of Albertania and Timaviella belonging to Leptolyngbyaceae [30,40]. The three novel species of Plectolyngbya described in this study have very different habitats from that of P. hodgsonii (Table 2). The type of species of P. hodgsonii was collected from littoral mat samples of the coastal lake in the Larsemann Hills and Monolith Lake on the James Ross Island of the Antarctic [9]. Meanwhile, P. terrestris and P. koreana were collected from black spots on soil and stone walls in Seoul, Korea, which are highly arid and subaerophytic (Figure 1). P. salina was isolated from a saltern pond in Korea with hypersaline water, which was consistent with the habitat of the type of species of P. hodgsonii (Table 2). These results support the Plectolyngbya distributed various habitats of Plectolyngbya in the world [9].

Table 2.
The comparison of the morphological characters of Plectolyngbya species.
In the Synechococcales, less than 98.65% 16S rRNA similarity between the taxa was suggested as a novel species identification criterion [54]. In Plectolyngbya, P. hodgsonii was distinguished from Leptolyngbya sensu stricto with 3.7–4.2% 16S rRNA distance (or 95.8–96.3% similarity) [9]. In the present study, the 16S rRNA similarities in the Plectolyngbya supported the recognition of three genuine new species (Table 1). The maximum 16S rRNA similarities were found between P. salina FBCC-A1477 and P. hodgsonii ANT.LPR2.2 (98.12%), between P. terrestris FBCC-A1479 Plectonema sp. SAG 38.90 (98.61%), and between P. koreana FBCC-A1478 and Plectolyngbya sp. WJT66-NPBG17 (98.78%) (Table 1). Based on previous criterion, Plectolyngbya sp. WJT66-NPGB17 seemed to be the same species as P. koreana FBCC-A1478.
The 16S–23S ITS secondary structure showed differences among all of the Plectolyngbya in the D1–D1’, Box-B, and V3 helices. In the D1–D1’ helices, the terminal stem of P. hodgsonii was similar to that of P. terrestris and P. koreana, but the unilateral bulge was different. The total length of the Box-B helices was 44 bp in P. hodgsonii, followed by 41 bp in P. salina, 37 bp in P. terrestris, and 32 bp in P. koreana (Figure 9F–J). For the V3 helices, P. terrestris and P. koreana were identical, and P. salina was identical to P. hodgsonii in the number and content of the stems and loops. The 16S–23S ITS secondary structures support the identity of strains, similar to previous reports [55]. Polyphasic approaches should be adopted in order to solve a few structural differences between strains within P. hodgsonii [56].
Through the habitat, morphological, and molecular results, Plectolyngbya terrestris FBCC-A1479, P. koreana FBCC-A1478, and P. salina FBCC-A1477 are unique and novel species within the Plectolyngbya, Leptolyngbyaceae.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14121013/s1. Figure S1: Alignment of the 16S–23S rRNA internal transcribed spacer (ITS) region from Plectolyngbya hodgsonii, P. terrestris, P. koreana, and P. salina.
Author Contributions
D.-H.K.: original concept, isolated the strains, sample collection, microscopy analysis, and drafting and editing manuscript; N.-J.L.: culture experiments, electron microscopy, and microscopy analysis; J.-H.K.: sample collection and culture experiments; E.-C.Y.: analysis of the molecular data, contributed to the molecular interpretation, and revised and edited the manuscript; O.-M.L.: contributed to the morphological interpretation, and revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Environment Industry and Technology Institute (KEITI) through the project to make multi-ministerial national biological research resources more advanced, funded by Korea Ministry of Environment (MOE) (2021003420004), a grant from the National Institute of Biological Resources (NIBR), funded by MOE of the Republic of Korea (NIBR202002117, NIBR202102202), and Kyonggi University‘s Graduate Research Assistantship 2022.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We wish to thank Michael Guiry of AlgaeBase, for advising with the scientific names and etymology and Earl Kyu Park, for English revisions to improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whitton, B.A.; Potts, M. Introduction to the cyanobacteria. In Ecology of Cyanobacteria II; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–13. [Google Scholar]
- Lindemann, S.R.; Moran, J.J.; Stegen, J.C.; Renslow, R.S.; Hutchison, J.R.; Cole, J.K.; Dohnalkova, A.C.; Tremblay, J.; Singh, K.; Malfatti, S.A.; et al. The epsomitic phototrophic microbial mat of Hot Lake, Washington: Community structural responses to seasonal cycling. Front. Microbiol. 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Doğan, S.Ş.; Kocabaş, A. Metagenomic assessment of prokaryotic diversity within hypersaline Tuz Lake, Turkey. Microbiology 2021, 90, 647–655. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Nübel, U.; Muyzer, G. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Arch. Microbiol. 1998, 169, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Cyanobacteria in hypersaline environments: Biodiversity and physiological properties. Biodivers. Conserv. 2015, 24, 781–798. [Google Scholar] [CrossRef]
- Nägeli, C. Gattungen einzelliger algen, physiologisch und systematisch bearheitet. Neue Denkschriften Der Allgemeinen Schweizerischen Gesellschaft für Die Gesammten Naturwissenschaften. Allg. Schweiz. Nat. Ges. 1849, 10, 1–139. [Google Scholar]
- Gomont, M. Monographie des Oscillariées (Nostocacées Homocystées). Ann. Sci. Nat. Bot. Sér. 1892, 16, 91–264. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 3-Oscillatoriales. Arch. Hydrobiol. 1988, 80, 327–472. [Google Scholar]
- Taton, A.; Wilmotte, A.; Šmarda, J.; Elster, J.; Komárek, J. Plectolyngbya hodgsonii: A novel filamentous cyanobacterium from Antarctic lakes. Polar Biol. 2011, 34, 181–191. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. The halotolerance and phylogeny of cyanobacteria with tightly coiled trichomes (Spirulina Turpin) and the description of Halospirulina tapeticola gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 1265–1277. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Vazquez-Nion, D.; Rodriguez-Castro, J.; Lopez-Rodriguez, M.C.; Fernandez-Silva, I.; Prieto, B. Subaerial biofilms on granitic historic buildings: Microbial diversity and development of phototrophic multi-species cultures. Biofouling 2016, 32, 657–669. [Google Scholar] [CrossRef]
- Bornet, É.; Flahault, C. Revision des Nostocacées hétérocystées contenues dans les principaux herbiers de France (quatrième et dernier fragment). Ann. Des Sci. Nat. Bot. Septième Sér. 1886, 7, 177–262. [Google Scholar]
- Zammit, G.; Billi, D.; Albertano, P. The subaerophytic cyanobacterium Oculatella subterranea (Oscillatoriales, Cyanophyceae) gen. et sp. nov.: A cytomorphological and molecular description. Eur. J. Phycol. 2012, 47, 341–354. [Google Scholar] [CrossRef]
- Bornet, É.; Flahault, C. Revision des Nostocacées hétérocystées contenues dans les principaux herbiers de France (Troisième fragment). Ann. Sci. Nat. Bot. Sept. Sér. 1886, 5, 51–129. [Google Scholar]
- Janet, M. Westiellopsis prolifica, gen. et sp. nov., a new member of the Stigonemataceae. Ann. Bot. 1941, 5, 167–170. [Google Scholar] [CrossRef]
- Dvořák, P.; Hašler, P.; Poulíčková, A. Phylogeography of the Microcoleus vaginatus (Cyanobacteria) from Three Continents—A Spatial and Temporal Characterization. PLoS ONE 2012, 7, e40153. [Google Scholar] [CrossRef] [PubMed]
- Hašler, P.; Dvořák, P.; Johansen, J.R.; Kitner, M.; Ondřej, V.; Poulíčková, A. Morphological and molecular study of epipelic filamentous genera Phormidium, Microcoleus and Geitlerinema (Oscillatoriales, Cyanophyta/cyanobacteria). J. Czech Phycol. Soc. 2012, 12, 341–356. [Google Scholar] [CrossRef]
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: www.algaebase.org (accessed on 3 November 2022).
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Johansen, J.R.; Kovácik, L.; Casamatta, D.A.; Iková, K.F.; Kaštovský, J. Utility of 16S-23S ITS sequence and secondary structure for recognition of intrageneric and intergeneric limits within cyanobacterial taxa: Leptolyngbya corticola sp. nov. (Pseudanabaenaceae, Cyanobacteria). Nova Hedwig. 2011, 92, 283–302. [Google Scholar] [CrossRef]
- Vaz, M.G.M.V.; Andreote, A.P.D.; Malone, C.F.S.; Sant’Anna, C.L.; Barbiero, L.; Fiore, M.F. Pantanalinema gen. nov. and Alkalinema gen. nov.: Novel pseudanabaenacean genera (Cyanobacteria) isolated from saline-alkaline lakes. Int. J. Syst. Evol. Microbiol. 2015, 65, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Genuário, D.B.; de Souza, W.R.; Monteiro, R.T.R.; Sant Anna, C.L.; Melo, I.S. Amazoninema gen. nov., (Synechococcales, Pseudanabaenaceae) a novel cyanobacteria genus from Brazilian Amazonian rivers. Int. J. Syst. Evol. Microbiol. 2018, 68, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Perkerson, R.B.; Johansen, J.R.; Kovácik, L.; Brand, J.; Kaštovský, J.; Casamatta, D.A. A unique pseudanabaenalean (Cyanobacteria) genus Nodosilinea gen. nov. based on morphological and molecular data. J. Phycol. 2011, 47, 1397–1412. [Google Scholar] [CrossRef]
- Jahodářová, E.; Dvořák, P.; Hašler, P.; Poulíčková, A. Revealing hidden diversity among tropical cyanobacteria: The new genus Onodrimia (Synechococcales, Cyanobacteria) described using the polyphasic approach. Phytotaxa 2017, 326, 28–40. [Google Scholar] [CrossRef]
- Turicchia, S.; Ventura, S.; Komárková, J.; Komárek, J. Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize. 2. Diversity of oscillatorialean genera. Nova Hedwig. 2009, 89, 165–200. [Google Scholar] [CrossRef]
- Song, G.-F.; Jiang, Y.-G.; Li, R.-H. Scytolyngbya timoleontis, gen. et sp. nov. (Leptolyngbyaceae, Cyanobacteria): A novel false branching cyanobacteria from China. Phytotaxa 2015, 224, 72–84. [Google Scholar] [CrossRef]
- Sciuto, K.; Moro, I. Detection of the new cosmopolitan genus Thermoleptolyngbya (Cyanobacteria, Leptolyngbyaceae) using the 16S rRNA gene and 16S-23S ITS region. Mol. Phylogenet. Evol. 2016, 105, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, K.; Moschin, E.; Moro, I. Cryptic cyanobacterial diversity in the giant cave (Trieste, Italy): The new genus Timaviella (Leptolyngbyaceae). Cryptogam. Algol. 2017, 38, 285–323. [Google Scholar] [CrossRef]
- Zimba, P.V.; Huang, I.-S.; Foley, J.E.; Linton, E.W. Identification of a new-to-science cyanobacterium, Toxifilum mysidocida gen. nov. & sp. nov. (Cyanobacteria, Cyanophyceae). J. Phycol. 2017, 53, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier: Heidelberg, Germany, 2005; pp. 1–749. [Google Scholar]
- Gomont, M. Monographie des Oscillariées (Nostocacées homocystées). Ann. Sci. Nat. Bot. Sér. 1892, 15, 263–368. [Google Scholar]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.C.B.G.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Lee, N.-J.; Seo, Y.; Ki, J.-S.; Lee, O.-M. A study of newly recorded genus and species for aerial cyanobacteria Wilmottia murrayi (Oscillatoriales, Cyanobacteria) in Korea. Korean J. Environ. Biol. 2019, 37, 260–267. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeong, M.S.; Kim, D.-Y.; Her, S.; Wie, M.-B. Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2015, 90, 204–214. [Google Scholar] [CrossRef]
- Rippka, R.; Waterbury, J.; Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974, 100, 419–436. [Google Scholar] [CrossRef]
- Saw, J.H.W.; Schatz, M.; Brown, M.V.; Kunkel, D.D.; Foster, J.S.; Shick, H.; Christensen, S.; Hou, S.; Wan, X.; Donachie, S.P. Cultivation and complete genome sequencing of Gloeobacter kilaueensis sp. nov., from a lava cave in Kilauea Caldera, Hawaii. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, R. Die sapropelische Lebewelt. Ein Beitrag zur Biologic des Faulschlamms nattirlicher Gewässer. Verh. Naturh. Med. Ver. Heidelb. 1915, 13, 395–481. [Google Scholar]
- Zammit, G. Systematics and biogeography of sciophilous cyanobacteria; an ecological and molecular description of Albertania skiophila (Leptolyngbyaceae) gen. & sp. nov. Phycologia 2018, 57, 481–491. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Han, K. PseudoViewer3: Generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 2009, 25, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, D.; Voultsiadou, E.; Panteris, E.; Zervou, S.-K.; Hiskia, A.; Gkelis, S. Leptothoe, a new genus of marine cyanobacteria (Synechococcales) and three new species associated with sponges from the Aegean Sea. J. Phycol. 2019, 55, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Komárková-Legnerová, J.; Cronberg, G. New and recombined filamentous cyanophytes from lakes in South Scania, Sweden. Arch. Hydrobiol. 1992, 95, 21–31. [Google Scholar]
- Konstantinou, D.; Voultsiadou, E.; Panteris, E.; Gkelis, S. Revealing new sponge-associated cyanobacterial diversity: Novel genera and species. Mol. Phylogenet. Evol. 2021, 155, 1–17. [Google Scholar] [CrossRef]
- Chakraborty, S.; Maruthanayagam, V.; Achari, A.; Pramanik, A.; Jaisankar, P.; Mukherjee, J. Euryhalinema mangrovii gen. nov., sp. nov. and Leptoelongatus litoralis gen. nov., sp. nov. (Leptolyngbyaceae) isolated from an Indian mangrove forest. Phytotaxa 2019, 422, 58–74. [Google Scholar] [CrossRef]
- Pietrasiak, N.; Osorio-Santos, K.; Shalygin, S.; Martin, M.P.; Johansen, J.R. When Is a lineage a species? a case study in Myxacorys gen. nov. (Synechococcales: Cyanobacteria) with the description of two new species from the Americas. J. Phycol. 2019, 55, 976–996. [Google Scholar] [CrossRef]
- Lee, N.-J.; Seo, Y.; Ki, J.-S.; Lee, O.-M. Morphology and molecular description of Wilmottia koreana sp. nov. (Oscillatoriales, Cyanobacteria) isolated from the Republic of Korea. Phytotaxa 2020, 447, 237–251. [Google Scholar] [CrossRef]
- Kim, D.-H.; Choi, H.J.; Ki, J.-S.; Lee, O.-M. Pinocchia daecheonga sp. nov. (Synechococcales, Cyanobacteria) isolated from a Daecheong lake in Geum river, Republic of Korea. Phytotaxa 2021, 510, 135–147. [Google Scholar] [CrossRef]
- Mai, T.; Johansen, J.R.; Pietrasiak, N.; Bohunická, M.; Martin, M.P. Revision of the Synechococcales (Cyanobacteria) through recognition of four families including Oculatellaceae fam. nov. and Trichocoleaceae fam. nov. and six new genera containing 14 species. Phytotaxa 2018, 365, 1–59. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.-S.; Park, S.-C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.H.; Kováčik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef]
- da Silva Malone, C.F.; Rigonato, J.; Laughinghouse, H.D.; Schmidt, E.C.; Bouzon, Z.L.; Wilmotte, A.; Fiore, M.F.; Sant’Anna, C.L. Cephalothrix gen. nov. (Cyanobacteria): Towards an intraspecific phylogenetic evaluation by multilocus analyses. Int. J. Syst. Evol. Microbiol. 2015, 65, 2993–3007. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).