Abstract
Greece is situated in the East Mediterranean region and in the Balkan peninsula, i.e., a European biodiversity hotspot with high endemism in subterranean and freshwater fauna, highlighting the need to understand its biodiversity. A literature search was undertaken to present a checklist of cladocerans and copepods based on a compilation of published and current data, from 1892 up to 2022 from inland surfaces and subterranean water bodies from different regions of Greece. For Cladocera, 80 species were recorded (9 families with 35 genera). The most diverse families were Chydoridae (20 genera with 33 species) and Daphniidae (5 genera with 27 species). For copepoda, 134 taxa were recorded, in surface water bodies (12 families with 34 genera), subterranean water bodies (7 families with 27 genera), and parasitic copepods (3 families with 3 genera). The most diverse families in surface waters were Cyclopidae (15 genera with 41 taxa) and Diaptomidae (5 genera with 17 species), while those in subterranean waters were Cyclopidae (11 genera with 35 taxa) and Canthocamptidae (6 genera with 17 taxa). More species are expected to be discovered after sampling understudied regions, especially islands, as well as water bodies such as temporary pools, swamps, ditches, puddles, and the littoral parts of lakes, while molecular studies are needed to clarify various cases of complex taxonomy.
1. Introduction
Studies on the zooplankton community in Greece date back to the end of the 19th century [1,2] and a checklist of the rotifer community has recently been published in order to provide a baseline for future studies [3]. The first published studies concerning the zooplankton community (by Richard in 1892 and 1897) regarded copepods and cladocerans, and even included the first description of a new species, i.e., Arctodiaptomus steindachneri [2]. Several other publications sporadically followed, providing information for several lakes [4] and island areas (Kiefer 1928; 1938; Brian 1929; Chappuis 1929 from Zarfdjian and Economidis [5] and references therein [6]). In the mid-1900s, when zooplankton fauna of Greece was still poorly known, Stephanides [6,7,8,9] extensively studied the crustacean fauna of the island Corfu, while Pesce later focused on subterranean copepod fauna from both the islands and the Greek mainland in many publications from 1979 to 1986 [10,11,12,13,14,15,16,17]. More recently, Marrone et al. reported 46 taxa from the island of Crete [18] and 20 taxa from the Fthiotis area [19]. These studies demonstrate that biodiversity patterns can actually reflect the areas where taxonomists live and work, or go on holiday or conduct fieldwork [20,21,22,23]. For rotifers, i.e., another group of the zooplankton community, Fontaneto et al. [22] coined the term ‘rotiferologist effect’. In analogue, we could use the terms ‘copepodologist effect’ or/and the ‘effect of the cladoceran specialist’, which were identified in previous years in Greece. All these could improve the faunistic knowledge of a specific area and misleadingly identify low biodiversity in another underexplored area.
Furthermore, regional species diversity should be considered in a historical context, reflecting the taxonomic resolution that can vary with time [24]. Following the ‘specialist effect’, European experts identified European-like species from all over the world, ‘creating’ cosmopolitan species that were later identified to be species complexes [24]. Thus, reliable data on the distribution of all existing species are needed to understand diversity, biogeography, and ecosystem processes, as well as provide the base for future studies. The global revision of taxonomy should be conducted in close coordination with phylogenetic studies, including the modern approach of integrative taxonomy, combining morphology, ecology, and molecular analysis, which currently aim to achieve a complete biodiversity record of all species [25].
Cladocera are important components of freshwater ecosystems; they hold a keystone position in ecosystem functioning and are critical for management and restoration plans. They are mainly members of the zooplankton community of surface freshwaters with currently recognized 620–650 species, although the actual diversity is estimated to be 2–4 times higher [26]. There are many cases of extensive phenotypic plasticity and hybridization that hamper the taxonomic identity of several species that form species complexes.
Copepods can be found in almost all freshwater habitats from moist soils, leafpacks, groundwater, wetlands, and phytotelmata. They comprise a major part of the zooplankton biomass, playing a pivotal role in aquatic food webs, both as primary and secondary consumers, while they also serve as a major part of food for higher trophic levels. Approximately 2814 free-living species reside in freshwaters, but this number is expected to grow as other species complexes are discovered [24]. Furthermore, about 330 species of copepods in freshwaters are parasitic on fish and molluscs, while they also live as commensal epibionts on freshwater invertebrates [24].
An area of great interest regarding its biodiversity is the region of the Mediterranean basin which is regarded as a biodiversity hotspot, although its freshwater fauna is not well studied [27]. Greece, besides belonging to the Mediterranean regions, is also part of the Balkan Peninsula, a European biodiversity hotspot with high endemism in subterranean and freshwater fauna, hosting ancient lakes, thus highlighting the importance of understanding its biodiversity even more. Recently, updated zooplankton checklists (e.g., [3,28,29,30]) were published across the Balkan Peninsula in order for zooplankton fauna to be used for diversity conservation and ecological studies in light of the global threats faced by aquatic ecosystems. Herein, we present an up-to-date checklist for both cladocerans and copepods based on a compilation of published and current data from 1892 up to 2022.
2. Materials and Methods
Here, we provide data from surface inland water bodies [24 natural lakes, 14 reservoirs, 2 artificial urban ponds, a man-made water channel connecting lakes Mikri Prespa and Megali Prespa, a river (Aliakmon), a wetland (Kalodiki), and various small water bodies from different regions of Greece (Figure 1a)] and subterranean water bodies (e.g., wells, caves) of Greece (Figure 1b). Morphometric characteristics, trophic states, and salinity values for each water body, when available, are presented in Table S1.
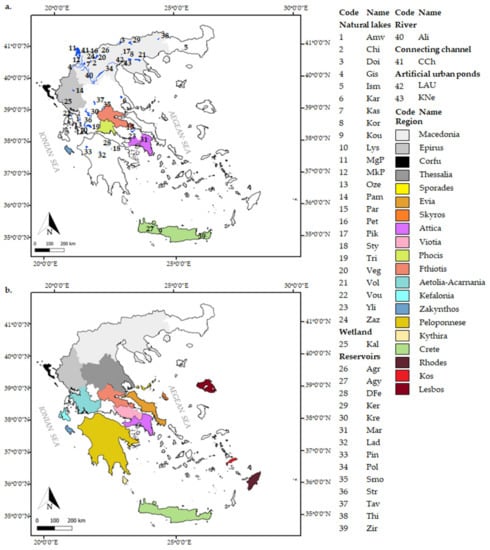
Figure 1.
Map of Greece showing (a) the locations of the surface water bodies and (b) the locations of the subterranean water bodies included in the study. Abbreviations: Amv: Amvrakia, Chi: Chimaditida, Doi: Doirani, Gis: Gistova, Ism: Ismarida, Kar: Karla, Kas: Kastoria, Kor: Koronia, Kou: Kournas, Lys: Lysimaxeia, MgP: Megali Prespa, MkP: Mikri Prespa, Oze: Ozeros, Pam: Pamvotis, Par: Paralimni, Pet: Petron, Pik: Pikrolimni, Sty: Stymfalia, Tri: Trichonis, Veg: Vegoritis, Vol: Volvi, Vou: Voulkaria, Yli: Yliki, Zaz: Zazari, Kal: Kalodiki, Agr: Agra, Agy: Agya, DFe: Doxa-Feneou, Ker: Kerkini, Kre: Kremasta, Mar: Marathona, Lad: Ladona, Pin: Pineiou, Pol: Polyfytos, Smo: Smokovo, Str: Stratos, Tav: Tavropou, This: Thisavros, Zir: Zirou, Ali: Aliakmon, CCh: connecting channel (between Megali and Mikri Prespa), KNe: Kipos nerou pond, LAU: Limnoula auth. Skyros is an island of the Sporades archipelago which is indicated separately due to the number of species recorded there for the first time.
The checklist of crustaceans recorded in inland surface or subterranean water bodies from Greece compiled herein was based on already-published data and data in the present study. A bibliographic review of crustaceans’ diversity was conducted using the databases Google Scholar and Web of Science, as well as the search words “crustacea”, “copepoda”, “cladocera”, “Greece”, and “diversity” during the entire period available in each database (retrieved during May 2022) and the National Archive of PhD Theses of Greece. The grey literature, including bachelor and master theses and technical reports, are also presented. Moreover, historic data from 1892 up to 1987 included in a previous checklist [5], which were not available in the above databases, are cited as Zarfdjian and Economidis [5] in Table S1. Studies with only genus-level identifications were not included in our dataset. The list of consulted works per water body/region is available in Table S1.
Data were sorted into an Excel file according to the inland surface water body (Table S2 for cladocerans and Table S3 for copepods) and the region for subterranean water bodies (Table S4). Currently valid species names, authorships, synonyms, and spellings were verified and updated using the World Register of Marine Species (WoRMS) [31]. When necessary, we checked the species identifications based on available pictures and updated the species identification of genus Diaphanosoma based on the literature [32].
For each species, the relative frequency of occurrence (i.e., the number of times a certain species occurred in all examined water bodies) was calculated. The species were then categorized as rare when the frequency of occurrence ranged up to 20%, moderate when the frequency of occurrence ranged between 20% and 50%, and frequent when the frequency of occurrence was greater than 50%. Moreover, a literature review was conducted for cladoceran and copepod’s functional traits (Tables S2–S4). The feeding type of Cladocera was subdivided into five classes (B-type for bosminids, C-type for chydorids and macrothricids, D-type for daphnids, S-type for sidids, and I-type for ilyocrypids) based on how they obtain their food [33,34]. The trophic group of Cladocera was divided to filter-feeders (feeding on algae, bacteria, or detritus) and carnivores for the raptorial Leptodora. The trophic group of Copepoda was divided into traditional herbivore, omnivore, and carnivore categories, with the addition of an omnivore–herbivore category to distinguish omnivore copepods that are more herbivorous [33]. Furthermore, the habitat preference is mentioned, i.e., littoral or pelagic, based on where each species is most likely to be found [33,34,35].
3. Results
3.1. Cladocera
The total number of Cladocera species reported from Greece was 80 (Table 1). These species were classified into 1 class (Branchiopoda), 1 superorder (Diplostraca), 3 orders (Anomopoda, Ctenopoda, and Haplopoda), 9 families, and 35 genera (Table 2). The most diverse family was Chydoridae with 20 genera and 33 species, followed by Daphniidae with 5 genera and 27 species (Figure 2). In lakes and reservoirs, for which more extensive data exist, Bosmina longirostris and Daphnia cucullata had the highest frequencies of occurrence, with 89% and 68%, respectively. All cladocerans were recorded in surface waters, with only two, Coronatella rectangula and Daphnia pulex, being recorded in subterranean water body wells in Attica [36] and Corfu [7,8,9], respectively. Although cladocerans are mainly known from surface water bodies, members of the chydorids are known to be accidentally found in wells and caves as well [37,38].

Table 1.
A list of cladocera species recorded in the studied water bodies of Greece (abbreviations according to Figure 1), * indicates species also reported from subterranean water bodies.

Table 2.
Taxonomy and number of cladoceran species per genus.
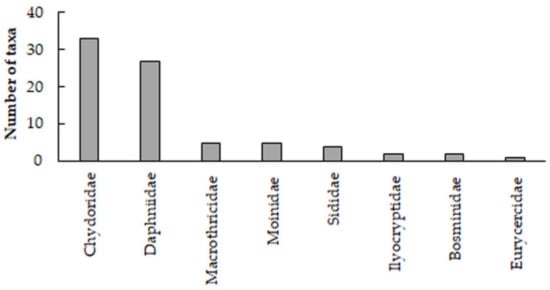
Figure 2.
Number of Cladocera species per family recorded in Greek waterbodies.
3.2. Copepoda
The total number of copepod taxa (species or subspecies) reported from Greece was 135; this included taxa recorded from surface water bodies, subterranean water bodies, and parasitic copepods.
3.2.1. Copepods from Surface Water Bodies
The total number of copepod species reported from surface inland water bodies was 78 (Table 3). These were classified into 1 class (Copepoda), 2 superorders (Gymnoplea and Podoplea), 3 orders (Calanoida, Cyclopoida, and Harpacticoida), 12 families, and 34 genera (Table 4). The most diverse family was Cyclopidae with 15 genera and 41 taxa, followed by Diaptomidae with 5 genera and 17 species and Canthocamptidae with 5 genera and 9 taxa (Figure 3). In lakes and reservoirs, for which more extensive data exist, Cyclops vicinus and Macrocyclops albidus had the highest frequencies of occurrence, with 46% and 43%, respectively.

Table 3.
A list of copepod taxa recorded in the studied surface water bodies of Greece (abbreviations according to Figure 1); * indicates species also reported from subterranean water bodies.

Table 4.
The taxonomy and number of copepod taxa per genus for surface water bodies.
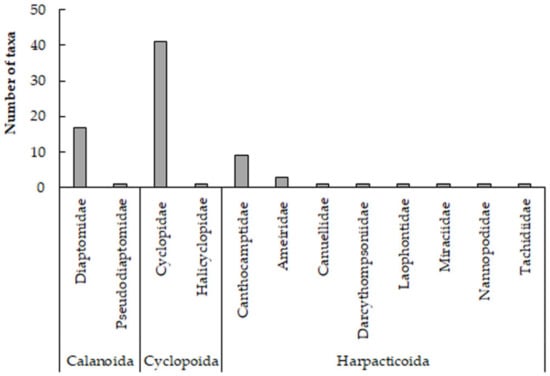
Figure 3.
The number of copepod taxa per family recorded in Greek surface water bodies.
3.2.2. Copepods from Subterranean Water Bodies
The total number of copepod species reported from subterranean inland water bodies was 70 (Table 5). These were classified into 1 class (Copepoda), 2 superorders (Gymnoplea and Podoplea), 3 orders (Calanoida, Cyclopoida, and Harpacticoida), 7 families, and 27 genera (Table 6). The most diverse family was Cyclopidae with 11 genera and 35 taxa, followed by Canthocamptidae with 6 genera and 17 taxa (Figure 4).

Table 5.
A list of copepod taxa recorded in the studied subterranean water bodies of Greece; * indicates species also reported from surface water bodies.

Table 6.
The taxonomy and number of copepod taxa per genus for subterranean water bodies.
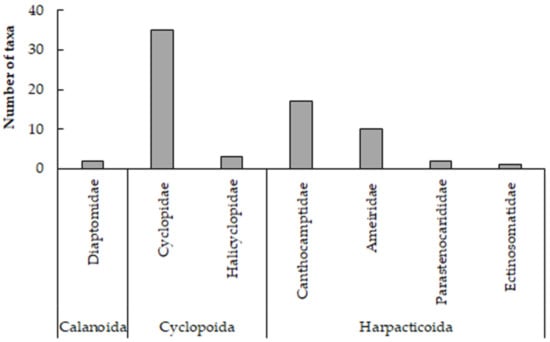
Figure 4.
The number of copepod taxa per family recorded in Greek subterranean water bodies.
Species mentioned without specifying the type (surface or subterranean) of the water body are presented in Table 7. Six more taxa, namely Occidodiaptomus gurneyi, Mixodiaptomus kupelwieseri, Halicyclops magniceps, Bryocamptus (Rheocamptus) zschokkei zschokkei, Elaphoidella eucharis, Elaphoidella varians were new records for the Greek fauna.

Table 7.
Taxa recorded in Zarfdjian and Economidis [5] without specifying the reference or if the taxa were recorded in surface or subterranean waterbodies. Underlined species have not been reported in the previous studies, n.s.: not specified.
3.2.3. Parasitic Copepods
The total number of parasitic copepods species was four (Table 8). These were classified into one class (Copepoda), one superorder (Podoplea), two orders (Siphonostomatoida and Cyclopoida), three families, and three genera (Table 8).

Table 8.
A list of parasitic copepod taxa recorded in Greece.
4. Discussion
4.1. Cladocera
For Cladocera, from the literature review, cases of misidentification were noted, which were not included in the present checklist. For example, Bythotrephes longimanus (Leydig, 1860) was recorded in Lake Koronia by Christomanos [40], but based on the photographs provided in reference to this species, its presence cannot be confirmed (the picture shows a copepod and in no other picture is Bythotrephes depicted). Another case concerns the prior records of Diaphanosoma brachyurum (Liévin, 1848) from 12 lakes (e.g., [41,42,43]) and Diaphanosoma lacustris (Kořínek, 1981) in Lake Karla and Pamvotis [44]. Subsequent studies led to the actual finding of other species in Lake Pamvotis, i.e., D. mongolianum (Lake Karla was dried out in the 1960’s), which are now mentioned in Table 1, and cases of lakes Doirani, Kastoria, Kerkini, Mikri Prespa, Pamvotis, Paralimni, Vegoritis, and Volvi were confirmed by Alexiou et al. [32] through the use of morphological and molecular analyses. This is generally the case for D. brachyurum which has been considered cosmopolitan and is the most recorded Diaphanosoma species worldwide due to inadequate knowledge surrounding the morphology, and this name has been erroneously used to refer to other species [32,45] and it cannot be located in many of those regions anymore [32,46] as other Diaphanosoma species are now recorded [47].
A case with complex taxonomy is that of Daphnia hyalina. Phenotypically and ecologically speaking, it is a highly variable species, but it was proposed to be synonymous with Daphnia longispina based on molecular data [48,49]. These findings are currently considered only a hypothesis which should be checked in subsequent studies based on a recent critical review of the genus Daphnia [25]. We present it here as a species following the taxonomy presented in WoRMS [31]. Based on our data, Daphnia hyalina is a species commonly recorded in the first studies (e.g., [4,6,41,50]), while subsequent studies recorded D. cucullata and/or Daphnia galeata in the same lakes. Daphnia hyalina is found to hybridize with D. cucullata and D. galeata, forming hybrids and further increasing the morphological variability [51,52]. It is also gradually replaced by D. galeata during periods of eutrophication [48]. The future identification of Daphnia species in faunistic studies, combining morphological and molecular data, should be performed to verify the occurrence of these confusing taxonomical identities.
4.2. Copepoda
Copepod species, commonly recorded during the first few studies performed in Greece, were not recently found in the same water bodies; this could be explained by species misidentification due to a lack of proper and updated taxonomic keys, the subsequent identification of species complexes, or species replacement. From the literature review, cases of misidentification of copepod species were noted. For example, Macrocyclops fuscus was recorded in Lake Koronia by Christomanos [40] (as Cyclops fuscus), but based on the published photographs in reference to this species, its presence cannot be confirmed based on the morphology of the furcal rami. Based on the photographs given by Christomanos [40], this individual could be C. vicinus, which is known to have established populations in Lake Koronia (e.g., [42,53]). Another case is the calanoid Limnocalanus macrurus (Sars G.O., 1863) which is recorded in Lake Stratos [54], but is probably a misidentification since it is a marine–brackish species [31]. Invasions into freshwaters are recorded in lakes created at the margins of ice sheets after glaciers [48], while Stratos reservoir, situated in Acheloos River in western Greece, has low conductivity (<400 mS/m) [54]; thus, the presence of L. macrurus is questionable.
Cases with complex taxonomy were also recorded. Stephanides [6] recorded in Corfu “Diaptomus serbicus = Diaptomus mirus” and Marrone et al. [19] recorded Diaptomus cf. serbicus in Fthiotis. Diaptomus serbicus (Gjorgjevic, 1907) is considered to be synonymous with Diaptomus mirus serbicus [55], and the accepted name is Diaptomus mirus (Lilljeborg, 1889), according to WoRMS [31], which represents a species complex [56]. Thus, in Table 3, D. mirus is presented in both Corfu and Fthiotis, awaiting further clarification. Another case is the Ecyclops serrulatus group. E. serrulatus was recorded in many lakes and regions of Greece from both surface and subterranean waters (Table 3). Based on recent studies, about 19 valid species belong to the serrulatus group, with 6 of them being reported from Europe [48]. Moreover, Cyclops agilis (Koch, 1938) is synonymous to Eucyclops agilis (Koch, 1938) [31] and is sometimes equated with E. serrulatus [57]. In Greece, Stephanides found E. agilis in Corfu [6] (mentioning “C. agilis-C. serrulatus s. restr.”) and in Attica [36]; we present these records in Table 3 since E. agilis is considered a valid species in WoRMS [31]. Molecular analysis should be performed in future studies to verify the occurrence of E. agilis and E. serrulatus taxa in Greece. Another case is the genus Thermocyclops; in the prior studies, Thermocyclops hyalinus was a commonly found cyclopoid, while Thermocyclops crassus was later recorded in the same lakes (Table 3). Despite mentioning both species as synonymous in many studies (e.g., [48,58]), WoRMS refer to both species as valid [31]. Thermocyclops crassus has also been confused with Thermocyclops oithonoides [58], a species also recorded in Greece. Future studies in water bodies with more than one species from the genus Thermocyclops will reveal the correct distribution of this genus in Greece.
Cases of calanoid species recorded in former studies but not found in the same water bodies in more recent studies were also recorded. For example, Arctodiaptomus dudichi, Arctodiaptomus stephanidesi, and A. steindachneri in Lake Trichonis were recorded by Koussouris (as mentioned by [5,59]); however they were not found in more recent studies (e.g., [60,61]). The same stands for Lake Koronia with Arctodiaptomus bacillifer [62], Arctodiaptomus salinus and Eudiaptomus vulgaris [4]; however, this lake has dried up several times (2002, 2007, 2009, and 2014) [53] and some species did not re-establish populations.
Copepods from subterranean water bodies were mainly studied in the past. Their research started with Lindberg [63,64] and Chappuis [65] and was continued by Stephanides [36]. Later, a series of studies from 1977 to 1986 by de Giuseppe L. Pesce and Domenico Maggi and their team focused on both the Greek islands and the Greek mainland, while recently these habitats have been understudied, with only two studies [66,67] being published over the last few decades. So, additional species are expected to be found by future studies, considering that subterranean waters have not been extensively studied in many parts of Greece. It should also be acknowledged that many of these studies have new descriptions of species or subspecies (e.g., [12,13,14,15,16,66]), and that even new descriptions of endemic species may arise.
The subterranean fauna included taxa characteristic of the surface waters (e.g., Acanthocyclops robustus–vernalis species complex, A. bacillifer, and Neolovenula alluaudi) which can accidentally be found in the upper layers of subterranean waters [16]. This knowledge can be used to better understand the dispersal of these species to nearby water bodies.
Parasitic copepods are understudied in Greece. Only one study [39] was found during the literature review reporting Caligus apodus as a parasite to Chelon ramada (Risso, 1827), while Ergasilus lizae to Mugil cephalus (Linnaeus, 1758). Lernaea cyprinacea, and Ergasilus sieboldin were mentioned in the previous checklist [5], based on unpublished studies, but are known as serious fish pests, causing mass mortality and significant commercial losses in freshwater aquaculture [24]. However, parasitic copepods were also found in other lakes [e.g., Kourna and Paralimni (personal data)] without being identified.
4.3. Overall
The diversity of both cladoceran and copepod communities from surface inland water bodies is most probably underestimated due to various factors. First, the sampling efforts are not the same for all types of habitats across Greece. Over the last few decades, many research studies have focused on the monitoring of surface water bodies, especially lakes, and the ways that they function (e.g., [53,59,68,69,70]), while other important habitats such as temporary pools, swamps, ditches, and puddles were studied only in some parts of Greece (e.g., islands such as Corfu and Zakynthos [6,7]; Crete [18]; and regional units such as Attica [36], Epirus [6], and Fthiotis [19]). Thus, many regions have not been explored, especially islands, and rivers and temporary waterbodies are not well studied either; therefore, additional species are expected to be found in future studies. Even when lakes are studied, samplings are usually conducted at the deepest part of the lakes [71,72], so littoral species have been underestimated. Studies with long timeseries are missing, while many studies have sparce samplings, usually limited to the summer–autumn season of lakes (e.g., [73]). Furthermore, considering that subterranean and especially parasitic copepods are vastly understudied in Greece, the gab of knowledge concerning their diversity is even higher. Another factor of species richness underestimation can be connected to the presence of cryptic taxa, revealed through the application of analyses in molecular approaches using integrative taxonomy. Thus, future studies should focus on long-term monitoring, with sampling stations covering different habitats or unexplored water habitats to increase the species richness of Cladocera and Copepoda in Greece.
Another aspect regarding future studies is related to the functional traits of organisms. Based on data availability, cladocerans are more extensively studied compared to copepods [33], where data for groundwater copepods are scarce (Tables S2–S4). This knowledge gap is bigger for species without global distribution or rare species (usually littoral) which do not commonly dominate zooplankton communities.
5. Conclusions
The present study provides an updated checklist of cladocerans and copepods from inland water bodies of Greece based on a compilation of published and current data from 1892 up to 2022. The results identify the knowledge gap that exists concerning the Greek crustacean planktonic fauna associated with specific taxa (e.g., Harpacticoida), habitats (e.g., littoral zone, subterranean, and small water bodies), and regions, emphasizing the importance of its study under the light of a global increase in invasive species and the concomitant threat faced by aquatic ecosystems due to changes in species composition and food web relationships. Future studies should use the current checklist as a reference and set the goals for biodiversity exploration in understudied areas and habitats, as well as disentangle taxonomic cases that are locally and globally identified as problematic. Following this, ecological studies should be performed to identify the keystone species which are important for the functioning, restoration, and management of ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14110997/s1. Table S1: Water bodies’ characteristics; Table S2: Cladocera’s raw data; Table S3: Surface Copepoda’ s raw data; Table S4: Subterranean Copepoda’ s raw data. References [74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123] are cited in the supplementary materials.
Author Contributions
Conceptualization, E.M. and G.S.; formal analysis, G.S.; investigation, E.M., G.S. and P.K.; resources, E.M.; data curation, E.M. and G.S.; writing—original draft preparation, E.M., G.S. and P.K.; writing—review and editing, E.M., G.S. and P.K.; visualization, G.S.; supervision, E.M.; project administration, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Richard, J. Animaux Inférieurs, Notamment Entomostracés, Recueillis Par M. Le Prof. Steindachner Dans Les Lacs de La Macédoine. Ann. K.R. Nat. Hist. Hofmus. Wien. 1892, 7, 151–153. [Google Scholar]
- Richard, J. Entomostracés, Recueillis par M. Le Directeur Steindachner dans les Lacs de Janina et de Scutari. Ann. Nat. Mus. Wien 1897, 12, 63–66. [Google Scholar]
- Stamou, G.; Savva, A.; Demertzioglou, M.; Michaloudi, E. Diversity of Rotifera (Subclass: Monogononta) from Inland Water Bodies in Greece: An Updated Checklist. Diversity 2022, 14, 451. [Google Scholar] [CrossRef]
- Georgevitch, J. Les Organisms du Plankton des Grands Lacs de la Peninsula Balkanique. Mem. Société Zool. Fr. 1907, 20, 5–19. [Google Scholar]
- Zarfdjian, M.H.; Economidis, P.S. Listes Provisoires des Rotifères, Cladocéres et Copépodes des Eaux Continentales Grecques. Biol. Gallo-Hell. 1989, 15, 129–146. [Google Scholar]
- Stephanides, T. Synoptic Notes on the Fresh-Water Organisms of Certain Regions of Macedonia and Central Greece. Prakt. Hell Inst. 1948, 2, 205–213. [Google Scholar]
- Stephanides, T. A Seasonal Survey of the Entomostraca in Three Corfu Ponds. Prakt. Hell. Hydrobioligal Inst. 1960, 7, 5–20. [Google Scholar]
- Stephanides, T. Some Notes on the Entomostraca of Corfu Greece, after Interval of 23 Years. Prakt. Hell. Hydrobioligal Inst. 1960, 7, 3–10. [Google Scholar]
- Stephanides, T. Some Further Notes on the Entomostraca of Corfu, Greece, after an Interval of 25 Years. Prakt. Hell. Hydrobioligal Inst. 1964, 9, 1–12. [Google Scholar]
- Pesce, G.L.; Maggi, D. Un nouveau Cyclopide des eaux souterraines phréatiques de Gréce: Acanthocyclops (Megacyclops) dussarti n.sp. (Crustacea, Copepoda). Vie Milieu 1977, 27, 77–82. [Google Scholar]
- Pesce, G.L. A New Cyclopid from Subterranean Phreatic Waters of Greece Acanthocyclops (Acanthocyclops) cephallenus n. sp. (Crustacea: Copepoda). Vie Milieu 1978, 28–29, 77–82. [Google Scholar]
- Pesce, G.L. Some Harpacticoids from Subterranean Waters of Greece (Crustacea: Copepoda). Bolletino Zool. 1981, 48, 263–276. [Google Scholar] [CrossRef]
- Pesce, G.L. A New Nitocrella Chappuis 1923 from Phreatic Waters of Skyros Island, Greece. Senckenberg. Biol. 1981, 62, 399–403. [Google Scholar]
- Pesce, G.L. A New Nitocrella from Phreatic Subterranean Waters of Rhodes, Greece (Crustacea Copepoda, Harpacticoida). Rev. Suisse Zool. 1983, 90, 263–267. [Google Scholar]
- Pesce, G.L. A Revised Key to the Nitocrella Species of the Hirta-Group, Including the Description of a New Species from Phreatic Waters of Lesbos, Greece (Copepoda Harpacticoida: Ameiridae). Bull. Zool. Mus. 1983, 9, 109–113. [Google Scholar]
- Pesce, G.L. A New Harpacticoid from Phreatic Waters of Lesbos, Greece, and Notes on the “Rassenkreise” of Elaphoidella elaphoides (Chappuis) (Copepoda: Ameiridae). Rev. Suisse Zool. 1985, 92, 605–612. [Google Scholar] [CrossRef]
- Pesce, G.L. Arpacticoidi Stigobionti di Grecia (Crustacea: Copepoda). Zool. Ellenica. 1986, 25–34. [Google Scholar]
- Marrone, F.; Giuseppe, A.; Stoch, F.; Pieri, V.; Alonso, M.; Dretakis, M.; Naselli-Flores, L. An Account on the Non-Malacostracan Crustacean Fauna from the Inland Waters of Crete, Greece, with the Synonymization of Arctodiaptomus piliger Brehm, 1955 with Arctodiaptomus alpinus (Imhof, 1885) (Copepoda: Calanoida). Limnetica 2019, 38, 167–187. [Google Scholar] [CrossRef]
- Marrone, F.; Arculeo, M.; Georgiadis, C.; Stoch, F. On the Non-Malacostracan Crustaceans (Crustacea: Branchiopoda, Copepoda, Ostracoda) from the Inland Waters of Fthiotida (Greece). Biogeogr-J. Integr. Biogeogr. 2019, 34, 87–99. [Google Scholar] [CrossRef]
- Dumont, H.J. Biogeography of Rotifers. Hydrobiologia 1983, 104, 19–30. [Google Scholar] [CrossRef]
- Segers, H.; De Smet, W.H. Diversity and endemism in Rotifera: A review, and Keratella bory de St Vincent. Biodivers Conserv. 2008, 17, 303–316. [Google Scholar] [CrossRef]
- Fontaneto, D.; Barbosa, A.M.; Segers, H.; Pautasso, M. The ‘Rotiferologist’ Effect and Other Global Correlates of Species Richness in Monogonont Rotifers. Ecography 2012, 35, 174–182. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J. Does the World Need Faunists? Based on Rotifer (Rotifera) Occurrence Reflections on the Role of Faunistic Research in Ecology. Int. Rev. Hydrobiol. 2019, 104, 49–56. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Defaye, D. Global Diversity of Copepods (Crustacea: Copepoda) in Freshwater. Hydrobiologia 2008, 595, 195–207. [Google Scholar] [CrossRef]
- Kotov, A.A. A Critical Review of the Current Taxonomy of the Genus Daphnia OF Müller, 1785 (Anomopoda, Cladocera). Zootaxa 2015, 3911, 184–200. [Google Scholar] [CrossRef]
- Forró, L.; Korovchinsky, N.M.; Kotov, A.A.; Petrusek, A. Global Diversity of Cladocerans (Cladocera; Crustacea) in Freshwater. Hydrobiologia 2007, 595, 177–184. [Google Scholar] [CrossRef]
- Darwall, W.; Carrizo, S.; Numa, C.; Barrios, V.; Freyhof, J.; Smith, K. Freshwater Key Biodiversity Areas in the Mediterranean Basin Hotspot: Informing Species Conservation and Development Planning in Freshwater Ecosystems, 1st ed.; IUCN: Cambridge, UK; Malaga, Spain, 2014; pp. 1–100. [Google Scholar]
- Shumka, S. Checklist of Rotifer Species from Albania (Phylum Rotifera). Opusc. Zool. Bp. 2021, 52, 99–109. [Google Scholar] [CrossRef]
- Tasevska, O.; Guseska, D.; Kostoski, G. A Checklist of Monogonont Rotifers (Rotifera: Monogononta) of Lake Ohrid, Republic of Macedonia. Acta Zool. Bulg. Suppl. 2019, 13, 57–62. [Google Scholar]
- Iepure, S.; Bădăluţă, C.A.; Moldovan, O.T. An Annotated Checklist of Groundwater Cyclopoida and Harpacticoida (Crustacea, Copepoda) from Romania with Notes on Their Distribution and Ecology. Subterr. Biol. 2021, 41, 87. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. 2022. Available online: https://www.marinespecies.org (accessed on 31 August 2022).
- Alexiou, R.; Stamou, G.; Minoudi, S.; Tourli, F.; Tsartsianidou, V.; Triantafyllidis, A.; Michaloudi, E. The Genus Diaphanosoma (Diplostraca: Sididae) in Greece: Morphological and Molecular Assessment. Zootaxa 2021, 5082, 572–582. [Google Scholar] [CrossRef]
- Barnett, A.J.; Finlay, K.; Beisner, B.E. Functional Diversity of Crustacean Zooplankton Communities: Towards a Trait-Based Classification. Freshw. Biol. 2007, 52, 796–813. [Google Scholar]
- Rizo, E.Z.C.; Gu, Y.; Papa, R.D.S.; Dumont, H.J.; Han, B.P. Identifying Functional Groups and Ecological Roles of Tropical and Subtropical Freshwater Cladocera in Asia. Hydrobiologia 2017, 799, 83–99. [Google Scholar] [CrossRef]
- Walseng, B.; Hessen, D.O.; Halvorsen, G.; Schartau, A.K. Major Contribution from Littoral Crustaceans to Zooplankton Species Richness in Lakes. Limnol. Oceanogr. 2006, 51, 2600–2606. [Google Scholar] [CrossRef]
- Stephanides, T. A Note on the Entomostraca of the Athens Area with Some Observations of the Life—History of Moina rectirostris. Prakt. Hell. Hydrobioligal Inst. 1964, 9, 1–9. [Google Scholar]
- Dumont, H.J. Groundwater Cladocera: A Synopsis. Hydrobiologia 1987, 145, 169–173. [Google Scholar] [CrossRef]
- Brancelj, A.; Dumont, H.J. A Review of the Diversity, Adaptations and Groundwater Colonization Pathways in Cladocera and Calanoida (Crustacea), Two Rare and Contrasting Groups of Stygobionts. Fundam. Appl. Limnol. Arch. Fuer Hydrobiol. 2007, 168, 3–17. [Google Scholar] [CrossRef]
- Ragias, V.; Athanassopoulou, F.; Sinis, A. Parasites of Mugilidae spp Reared under Semi- Intensive and Intensive Conditions in Greece. Bull. Eur. Assoc. Fish Pathol. 2005, 25, 107–113. [Google Scholar]
- Christomanos, A. Zur Kenntnis Des Planktons Der Seen Griechenlands. II. Folia Bioch Biol. Greeca 1972, 4, 127–144. [Google Scholar]
- Popovska-Stankovič, O. Prilog Kon Poznavanje Kladocerite Od Dojranskoto Ezero. Izd. Instituta Piscic. Repub. Makedonia Skopje 1958, 2, 127–144. [Google Scholar]
- Serafimova-Hadžišče, J. Zooplankton in Some Lakes of Aegean Lake Zone. Fragm. Balc. 1974, 17, 165–168. [Google Scholar]
- Koussouris, T.S.; Diapoulis, A.C.; Photis, G.D. Evaluating the Trophic Status of a Shallow Polluted Lake, Lake Ioannina, Greece. Toxicol. Environ. Chem. 1991, 31, 303–313. [Google Scholar] [CrossRef]
- Kořínek, V. Diaphanosoma birgei n. sp. (Crustacea, Cladocera). A New Species from America and Its Widely Distributed Subspecies Diaphanosoma birgei ssp. lacustris n. ssp. Can. J. Zool. 1981, 59, 1115–1121. [Google Scholar] [CrossRef]
- Korovchinsky, N.M. Cladocera: Ctenopoda: Families Sididae, Holopediidae & Pseudopenilidae (Branchiopoda: Cladocera). Identification Guides to the Plankton and Benthos of Inland Waters, 1st ed.; Backhuys Publishers-Margraf Publishers GmbH: Weikerscheim, Germany, 2018; pp. 1–203. [Google Scholar]
- Lakatos, C.; Urabe, J.; Makino, W. Cryptic Diversity of Japanese Diaphanosoma (Crustacea: Cladocera) Revealed by Morphological and Molecular Assessments. Inland Waters 2015, 5, 253–262. [Google Scholar] [CrossRef]
- Karpowicz, M.; Świslocka, M.; Slugocki, L.; Czerniawski, R.; López, C.; Kornijów, R. Distribution of Diaphanosoma (Diplostraca: Sididae) Genus in Central Europe–Morphological and Molecular Approach. Eur. Zool. J. 2022, 89, 1115–1128. [Google Scholar] [CrossRef]
- Bledzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer: Berlin, Germany, 2016; pp. 1–918. [Google Scholar]
- Petrusek, A.; Hobæk, A.; Nilssen, J.P.; Skage, M.; ČErnỳ, M.; Brede, N.; Schwenk, K. A Taxonomic Reappraisal of the European Daphnia longispina complex (Crustacea, Cladocera, Anomopoda). Zool. Scr. 2008, 37, 507–519. [Google Scholar] [CrossRef]
- Popovska-Stankovic, O. Die Plankton-Production Des Dojran-Sees, Vom Aug. 1951 Bis Aug 1952. Inst. Piscic. RP Macédoine Skopje 1954, 1, 1–20. [Google Scholar]
- Benzie, J.A. The Systematics of Australian Daphnia (Cladocera: Daphniidae). Species Descriptions and Keys. Hydrobiologia 1988, 166, 95–161. [Google Scholar] [CrossRef]
- Wolf, H.G.; Mort, M.A. Inter-Specific Hybridization Underlies Phenotypic Variability in Daphnia Populations. Oecologia 1986, 68, 507–511. [Google Scholar] [CrossRef]
- Demertzioglou, M.; Antonopoulou, E.; Voutsa, D.; Kozari, A.; Moustaka-Gouni, M.; Michaloudi, E. MAPKs and HSPs’ Activation of a Natural Daphnia magna Population in a Man-Perturbed Lake: Implications of Ecological Significance. Water 2021, 13, 283. [Google Scholar] [CrossRef]
- Kehayias, G.; Chalkia, E.; Chalkia, S.; Nistikakis, G.; Zacharias, I.; Zotos, A. Zooplankton Dynamics in the Upstream Part of Stratos Reservoir (Greece). Biologia 2008, 63, 699–710. [Google Scholar] [CrossRef]
- Djordjević, J. Prilozi Za Poznavanje Slatkovodne Faune Balkan. Poluostrva II Makedon. Hidrahnide Glas Srp. Kralj. Akad. Nauka Beogr. 1907, 71, 123–150. [Google Scholar]
- Alfonso, G.; Stoch, F.; Marrone, F. An annotated checklist and bibliography of the Diaptomidae (Copepoda, Calanoida) of Italy, Corsica, and the Maltese islands. J. Limnol. 2021, 80, 43–58. [Google Scholar] [CrossRef]
- Alekseev, V.; Dumont, H.J.; Pensaert, J.; Baribwegure, D.; Vanfleteren, J.R. A Redescription of Eucyclops serrulatus (Fischer, 1851) (Crustacea: Copepoda: Cyclopoida) and Some Related Taxa, with a Phylogeny of the E. serrulatus-group. Zool. Scr. 2006, 35, 123–147. [Google Scholar] [CrossRef]
- Defaye, D.; Dussart, B.H.; Fernando, C.H.; Sarnita, A.S. On Some Species of the Genus Thermocyclops (Crustacea, Copepoda) from the Oriental Region. Can. J. Zool. 1987, 65, 3144–3153. [Google Scholar] [CrossRef]
- Doulka, E.; Kehayias, G. Spatial and Temporal Distribution of Zooplankton in Lake Trichonis (Greece). J. Nat. Hist. 2008, 42, 575–595. [Google Scholar] [CrossRef]
- Doulka, E.; Kehayias, G.; Chalkia, E.; Leonardos, I.D. Feeding Strategies of Atherina boyeri (Risso 1810) in a Freshwater Ecosystem. J. Appl. Ichthyol. 2013, 29, 200–207. [Google Scholar] [CrossRef]
- Kehayias, G.; Michaloudi, E.; Bexi, A. Aspects on the Seasonal Dynamics and the Vertical Distribution of the Crustacean Zooplankton Community and the Dreissena polymorpha Larvae in Lake Trichonis. Mediterr. Mar. Sci. 2004, 5, 19–27. [Google Scholar] [CrossRef]
- Kilikidis, S.; Kamarianos, A.; Photis, G.; Koussouris, T.; Karamanlis, X.; Ouzounis, K. Ecological Study on Lakes of Northern Greece Ag. Vassiliou, Doirani and Vistonis. Assumptions to Install a Station for Reproduction and Experimental Fishery. Sci. Ann. Vet. Fac. Univ. Thessalon. 1984, 22, 269–439. [Google Scholar]
- Lindberg, K. Cyclopides (Crustacés Copépodes) de La Grece II. Fragm. Balcan. Skopje 1955, 1, 189–195. [Google Scholar]
- Lindberg, K. Cyclopides (Crustaces Copepodes) de Crete avec une liste de crustaces divers recueillis dans le lac de Kourna. Acta 1955, 4, 97–120. [Google Scholar]
- Chappuis, P.A. Harpacticoides Recoltes En Crete Par M. K. Lindberg. Folia Balc. 1956, 3, 15–18. [Google Scholar]
- Cottarelli, V.; Bruno, M.C. First Record of Parastenocarididae (Crustacea, Copepoda, Harpacticoida) from Subterranean Freshwater of Insular Greece and Description of Two New Species. Int. J. Speleol. 1996, 25, 43–57. [Google Scholar] [CrossRef][Green Version]
- Popa, I.; Brad, T.; Vaxevanopoulos, M.; Giurginca, A.; Baba, Ş.C.; Iepure, S.; Plăiaşu, R.; Sarbu, S.M. Rich and Diverse Subterranean Invertebrate Communities Inhabiting Melissotrypa Cave in Central Greece. Trav. Inst. Spéol. «Émile Racovitza» 2019, 58, 65–78. [Google Scholar]
- Michaloudi, E.; Zarfdjian, M.; Economidis, P.S. The Zooplankton of Lake Mikri Prespa, Northwestern Greece. Hydrobiologia 1997, 351, 77–94. [Google Scholar] [CrossRef]
- Μichaloudi, E.; Moustaka-Gouni, M.; Pantelidakis, K.; Katsiapi, M.; Genitsaris, S. Plankton Succession in the Temporary Lake Koronia after Intermittent Dry-Out. Int. Rev. Hydrobiol. 2012, 97, 405–419. [Google Scholar] [CrossRef]
- Stefanidis, K.; Papastergiadou, E. Influence of Hydrophyte Abundance on the Spatial Distribution of Zooplankton in Selected Lakes in Greece. Hydrobiologia 2010, 656, 55–65. [Google Scholar] [CrossRef]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Trophic State Assessment Based on Zooplankton Communities in Mediterranean Lakes. Hydrobiologia 2019, 844, 83–103. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Moustaka-Gouni, M.; Michaloudi, E.; Bobori, D.C. Biogeographical Patterns of Freshwater Micro and Macroorganisms: A Comparison between Phytoplankton, Zooplankton and Fish in the Eastern Mediterranean. J. Biogeogr. 2010, 37, 1341–1351. [Google Scholar] [CrossRef]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. The Neglected Zooplankton Communities as Indicators of Ecological Water Quality of Mediterranean Lakes. Limnetica 2021, 40, 359–373. [Google Scholar] [CrossRef]
- Chalkia, E.; Zacharias, I.; Thomatou, A.A.; Kehayias, G. Zooplankton Dynamics in a Gypsum Karst Lake and Interrelation with the Abiotic Environment. Biologia 2012, 67, 151–163. [Google Scholar] [CrossRef]
- Danielidis, D.B.; Spartinou, M.; Economou-Amilli, A. Limnological Survey of Lake Amvrakia, Western Greece. Hydrobiologia 1996, 318, 207–218. [Google Scholar] [CrossRef]
- Bláha, M.; Hulák, M.; Slouková, J.; Těšitel, J. Molecular and Morphological Patterns across Acanthocyclops vernalis-robustus species Complex (Copepoda, Cyclopoida). Zool. Scr. 2010, 39, 259–268. [Google Scholar] [CrossRef]
- Shehu, M.; Serravalle, F.; Alfonso, G.; Moscatello, S.; Belmonte, G. The Alpine Gistova (Mount Gramos, Albania-Greece Border) Biodiversity of an Isolated Microcosm. Thalass. Salentina 2009, 32, 53–62. [Google Scholar]
- Koussouris, T.; Diapoulis, A.; Fotis, G. For The Development and Drotection of Freshwater Resources in Greece. II Kastoria; Technical report; Institute of Oceanographic and Fisheries Research: Kastoria, Greece, 1985. [Google Scholar]
- Petkovski, S. Über Die Plankton-Cladoceren Des Kastorias-Sees in NW Griechenland (Crustacea, Anomopoda). Scopolia 1992, 26, 1–22. [Google Scholar]
- Matzafleri, N.; Psilovikos, A.; Sentas, A. Zooplankton Population Seasonal Variations in Relation to Nutrients. Case Study of Lake Kastoria, Western Macedonia, Greece. Fresenius Environ. Bull. 2017, 26, 1318–1324. [Google Scholar]
- Kilikidis, S.; Kamarianos, A.; Karamanlis, X.; Dellis, S.; Kousouris, T.; Fotis, G. Water Quality and Trophic Status Evaluation of the Polyphyto Reservoir, N. Greece. Toxicol. Environ. Chem. 1992, 36, 169–179. [Google Scholar] [CrossRef]
- Politou, C.Y.; Economidis, P.S.; Sinis, A.I. Feeding Biology of Bleak, Alburnus alburnus, in Lake Koronia, Northern Greece. J. Fish Biol. 1993, 43, 33–43. [Google Scholar] [CrossRef]
- Michaloudi, E.; Kostecka, M. Zooplankton of Lake Koroneia (Macedonia, Greece). Biol. Bratisl. 2004, 59, 165–172. [Google Scholar]
- Michaloudi, E.; Moustaka-Gouni, M.; Gkelis, S.; Pantelidakis, K. Plankton Community Structure during an Ecosystem Disruptive Algal Bloom of Prymnesium parvum. J. Plankton Res. 2009, 31, 301–309. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Michaloudi, E.; Kormas, K.A.; Katsiapi, M.; Vardaka, E.; Genitsaris, S. Plankton Changes as Critical Processes for Restoration Plans of Lakes Kastoria and Koronia. Eur. Water 2012, 40, 43–51. [Google Scholar]
- Serafimoska-Hadžišče, J. Vertical Migrations of Zooplankton in Lake Prespa. Rev. Trav. Stat. Hydrobiol. Ohrid 1954, 2, 29–38. [Google Scholar]
- Chalkia, E.; Kehayias, G. Zooplankton and Environmental Factors of a Recovering Eutrophic Lake (Lysimachia Lake, Western Greece). Biologia 2013, 68, 459–469. [Google Scholar] [CrossRef]
- Katsiapi, M.; Michaloudi, E.; Moustaka-Gouni, M.; Lo, J.P. First Ecological Evaluation of the Ancient Balkan Lake Megali Prespa Based on Plankton. J. Biol. Res. 2012, 17, 51–56. [Google Scholar]
- Stathatos, P.; Barry, J.; Christomanou, M.; Christomanos, A. Beitrag Zur Planktonkunde Des Kleinen Prespa Sees in Mazedonien (Griechenland). Folia Biochem. Biol. Graeca 1972, 9, 12–26. [Google Scholar]
- Michaloudi, E. Dry Weights of the Zooplankton of Lake Mikri Prespa (Macedonia, Greece). Elgian J. Zool. 2005, 135, 223–227. [Google Scholar]
- Chalkia, E.; Kehayias, G. Zooplankton Community Dynamics and Environmental Factors in Lake Ozeros (Greece). Mediterr. Mar. Sci. 2013, 14, 32–41. [Google Scholar] [CrossRef][Green Version]
- Antonopoulos, A.; Kagalou, I.; Michaloudi, E.; Leonardos, I. Limnological Features of a Shallow Eutrophic Lake (Lake Pamvotis, Greece) with Emphasis on Zooplankton Community Structure. Oceanol. Hydrobiol. Stud. 2008, 37, 1–14. [Google Scholar]
- Gkenas, C.; Malavasi, S.; Leonardos, I. Diet and Feeding Habits of Economidichthys pygmaeus (Perciformes: Gobiidae) in Lake Pamvotis, NW Greece: Diet of Economidichthys pygmaeus. J. Appl. Ichthyol. 2012, 28, 75–81. [Google Scholar] [CrossRef]
- Gkenas, C.; Oikonomou, A.; Economou, A.; Kiosse, F.; Leonardos, I. Life History Pattern and Feeding Habits of the Invasive Mosquitofish, Gambusia holbrooki, in Lake Pamvotis (NW Greece). J. Biol. Res. 2012, 17, 121–136. [Google Scholar]
- Chrisafi, E.; Kaspiris, P.; Katselis, G. Feeding Habits of Sand Smelt (Atherina boyeri, Risso 1810) in Trichonis Lake (Western Greece). J. Appl. Ichthyol. 2007, 23, 209–214. [Google Scholar] [CrossRef]
- Doulka, E.; Kehayias, G. Seasonal Vertical Distribution and Diel Migration of Zooplankton in a Temperate Stratified Lake. Biologia 2011, 66, 308–319. [Google Scholar] [CrossRef]
- Kehayias, G.; Tzavali, A.; Gini, M.; Michopoulou, E.; Tsounis, L. Fish Predation in the Proximity of Purse Seine Fishing Lights: The Case of Atherina boyeri (Actinopterygii: Atheriniformes: Atherinidae) in a Greek Lake. Acta Ichthyol. Piscat. 2018, 48, 51–60. [Google Scholar]
- Muller, H. Zur Kenntnis Des Planktons Der Seen Griechenlands, III. Das Zooplankton Des Vengoritis Sees. Folia Biochem. Biol. Graeca 1976, 13, 67–90. [Google Scholar]
- Zarfdjian, M.H.; Vranovský, M.; Economidis, P.S. Les Invertébrés Planctoniques du Lac Volvi (Macédoine, Grèce). The Planktonic Invertebrates of Lake Volvi (Macedonia, Greece). Int. Rev. Gesamten Hydrobiol. Hydrogr. 1990, 75, 403–412. [Google Scholar] [CrossRef]
- Kleanthidis, P.K.; Sinis, A.I. Feeding Habits of the Macedonian Shad, Alosa macedonica (Vinciguerra, 1921) in Lake Volvi (Greece): Seasonal and Ontogenetic Changes. Isr. J. Zool. 2001, 47, 213–232. [Google Scholar] [CrossRef]
- Stephanides, T. A Short Note on the Entomostraca of Lake Yliki (Likeri) and of Lake Marathon. Prakt. Hell. Hydrobioligal Inst. 1964, 9, 1–6. [Google Scholar]
- Klossas, A. A Study on the Hydrobiology of the Lake Kerkini Near Serres, 1st ed.; Ministry of Agriculture: Athens, Greece, 1975; pp. 5–61. [Google Scholar]
- Giapis, A.J. Ecology of the Lepomis gibbosus (L.). Kerkini Lake. Ph.D. Thesis, School of Foresty and Natural Enviroment, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2003. [Google Scholar]
- Kagalou, I.I.; Kosiori, A.; Leonardos, I.D. Assessing the Zooplankton Community and Environmental Factors in a Mediterranean Wetland. Environ. Monit. Assess. 2010, 170, 445–455. [Google Scholar] [CrossRef]
- Zarfdjian, M.H.; Michaloudi, E.; Bobori, D.C.; Mourelatos, S. Zooplankton Abundance in the Aliakmon River, Greece. Belg. J. Zool. 2000, 130, 31–36. [Google Scholar]
- Karaytug, S.; Boxshall, G.A. The Paracyclops fimbriatus-Complex (Copepoda, Cyclopoida): A Revision. Zoosystema 1998, 20, 563–602. [Google Scholar]
- Brehm, V. Calanoide Kopepoden Und Cladoceren Aus Kreta. Fragm. Balc. 1955, 17, 149–155. [Google Scholar]
- Pesce, G.L. Stygobiological Researches in Subterranean Waters of Lesbos (Greece) and Description of Stygonitocrella petkovski n. sp. Fragm. Balc. 1985, 12, 125–139. [Google Scholar]
- Pesce, G.L.; Maggi, D. Cyclopides et Calanoïdes Des Eaux Phréatiques de La Grèce Méridionale et Insulaire (Crutacea : Copepoda). Ecol. Mediterr. 1981, 7, 163–182. [Google Scholar] [CrossRef]
- Maggi, D.; Pesce, G.L. Cyclopides Des Eaux Souterraines Phréatiques de La Grèce Du Nord (Crustacea: Copepoda). In Proceedings of the 1r Symposium International sur la Zoogéographie et l’Ecologie de la Grèce et des Régions Avoisinantes, Athenes, Greece, April 1978. [Google Scholar]
- Pesce, G.L.; Maggi, D.; Ciocca, A.; Argano, R. Biological Researches on the Subterranean Phreatic Waters of Northern Greece. In Proceedings of the 1r Symposium International sur la Zoogeographie et LECOLOGIE de la grece et des Regions Avoisinantes, Athenes, Greece; 1979. [Google Scholar]
- Pesce, G.L. The Occurrence of Metacyclops subdolus Kiefer (Crustacea: Copepoda) in Subterranean Waters of Greece with Remarks on Its Systematic Status. Int. J. Speleol. 1978, 10, 179–183. [Google Scholar] [CrossRef][Green Version]
- Dodson, S. Species Richness of Crustacean Zooplankton in European Lakes of Different Sizes. Int. Ver. Für Theor. Angew. Limnol. Verhandlungen 1991, 24, 1223–1229. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Špoljar, M.; Zhang, C.; Pronin, M. Zooplankton Functional Traits as a Tool to Assess Latitudinal Variation in the Northern-Southern Temperate European Regions during Spring and Autumn Seasons. Ecol. Indic. 2020, 117, 106629. [Google Scholar] [CrossRef]
- Lapesa, S.; Snell, T.W.; Fields, D.M.; Serra, M. Selective Feeding of Arctodiaptomus salinus (Copepoda, Calanoida) on Co-Occurring Sibling Rotifer Species. Freshw. Biol. 2004, 49, 1053–1061. [Google Scholar] [CrossRef]
- Brucet, S.; Compte, J.; Boix, D.; López-Flores, R.; Quintana, X.D. Feeding of Nauplii, Copepodites and Adults of Calanipeda aquaedulcis (Calanoida) in Mediterranean Salt Marshes. Mar. Ecol. Prog. Ser. 2008, 355, 183–191. [Google Scholar] [CrossRef]
- Krztoń, W.; Kosiba, J. Variations in Zooplankton Functional Groups Density in Freshwater Ecosystems Exposed to Cyanobacterial Blooms. Sci. Total Environ. 2020, 730, 139044. [Google Scholar] [CrossRef]
- Fryer, G. The Food of Some Freshwater Cyclopoid Copepods and Its Ecological Significance. J. Anim. Ecol. 1957, 26, 263–286. [Google Scholar] [CrossRef]
- Rylov, V.M. Fauna of the USSR. Crustaceans. Freshwater Cyclopoida, 1st ed.; Nauka: Moscow, Russia, 1948; pp. 1–312. (In Russian) [Google Scholar]
- Dussart, B. Les Copépodes des Eaux Continentáles. II. Cyclopoides et Biologie, 1st ed.; N. Boubee & Cie: Paris, France, 1969; pp. 1–292. [Google Scholar]
- Dussart, B. Les Copépodes des Eaux Continentáles. I. Calanoides et Harpacticoides, 1st ed.; N. Boubee & Cie: Paris, France, 1967; pp. 1–500. [Google Scholar]
- Sarvala, J. Effect of Temperature on the Duration of Egg, Nauplius and Copepodite Development of Some Freshwater Benthic Copepoda. Freshw. Biol. 1979, 9, 515–534. [Google Scholar] [CrossRef]
- Sarvala, J. Ecology and Role of Benthic Copepods in Northern Lakes. J. Mar. Syst. 1998, 15, 75–86. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).