Abstract
Madagascar hosts a great diversity of bird species. This study focuses on the description of the diversity and prevalence of blood parasites (Haemosporida, trypanosomes and filarioid nematodes) in 131 blood samples of 14 species of Corvoidea, namely vangas (Vangidae), Coracina cinerea (Campephagidae), Dicrurus forficatus (Dicruridae) and Terpsiphone mutata (Monarchidae) found in primary rainforests on Madagascar. Blood parasites were detected using both molecular and microscopic methods. Multiplex PCR was used to detect mixed haemosporidian infections and nested PCR was used to describe a 479 bp fragment of the haemosporidian cytochrome b (cytb) gene. Furthermore, a 770 bp SSU rRNA fragment of trypanosomes, and, for microfilariae, a 690 bp fragment of 28S rRNA, as well as a 770 bp fragment of 28S rRNA, were amplified for identification using nested PCRs. Phylogenetic analyses were carried out for all sequences obtained from all blood parasite taxa. Over half of the samples (54.2%; n = 71) were infected with Haemosporida, whereas only 21.4% (n = 28) were infected with Trypanosoma and 5.3% (n = 7) contained filarioid nematode DNA. Fourteen of 56 blood smears contained some of the above-mentioned parasite taxa. The results corroborate the great diversity of blood parasites in the different bird species studied, especially in vangas. Vangas had the greatest diversity of parasites found, as well as the highest number of multiple infections, which may be due to their morphological diversity and resulting habitat use. Fifteen haemosporidian lineages, seven Trypanosoma and five filarioid nematode isolates were newly discovered in the avian species studied, particularly in the vangas. Members of the other Corvoidea families on Madagascar showed a lower susceptibility for avian haemosporidian parasites than vangas, which could be attributed to possible resistance against those parasites. The study confirmed the host specificity of some Haemosporida and microfilariae; however, it demonstrated that this was not the case for Trypanosoma.
1. Introduction
The island of Madagascar is classified as an important biodiversity hotspot [1]. This is not due to a particularly high number of species, but to an extremely high proportion of endemic species. For example, Madagascar harbors a total of 292 bird species, of which 108 are reported to be strictly endemic to this island [2]. The most prominent bird family endemic to Madagascar, the Vangidae, are an excellent example of adaptive radiation. Soon after colonizing the island, a single ancestral species showed explosive bursts of diversification, evolving striking differences in bill morphology that allowed exploitation of the extremely varied climatic conditions and habitats of the island [3,4]. Today, 15 genera of primarily insectivorous Vangidae exist of which 12 genera are monospecific [2]. Besides the vangas, most species of the superfamily Corvoidea present in Madagascar are also endemic. Terpsiphone paradise flycatchers (Monarchidae) are a globally widespread and highly speciose genus of passerines occurring over most of sub-Saharan Africa, southern and eastern Asia, the Philippines, and the western Indian Ocean islands [5]. Several of the Terpsiphone species are migratory. Three non-migratory species are endemic to the western Indian Ocean and are split into eight subspecies found amongst the different islands, with the Madagascar paradise flycatcher Terpsiphone mutata mutata being endemic to Madagascar [2]. The drongos (Dicruridae) include only the genus Dicrurus, comprising 21 insectivorous species [6]. All members of the family are morphologically highly homogenous being of similar size and showing a glossy black coloration and a more or less forked tail [7]. The Malagasy region hosts four species, each endemic to a certain island. One subspecies of the crested drongo Dicrurus forficatus forficatus is strictly endemic to Madagascar [2]. The Campephagidae represent an Old-World clade of tropical birds with more than 70 species [8]. The genus Coracina is the most diversified (50 species) with many species being endemic to small and remote islands. In contrast to drongos, Coracina species are rather disparate morphologically [9]. Three species occur in the Malagasy region of which only the ashy cuckoo-shrike Coracina cinerea is endemic to Madagascar [2]. The vangas, Madagascar paradise flycatcher, crested drongo and the ashy cuckoo-shrike can form mixed-species flocks [10].
Studies on blood parasites of the above mentioned Corvoidea species are rare. Morphological studies [11,12,13,14] have emphasized the identification of all kinds of blood parasites infecting birds (Haemosporida, trypanosomes and filarioid nematodes), whereas more recent molecular studies have focused mainly on the detection and description of haemosporidian parasites [15,16]. The order Haemosporida includes vector-borne protozoan parasites of the four genera Plasmodium, Haemoproteus, Leucocytozoon and Fallisia. Being the ancestors of malaria parasites, including human malaria, and because of their importance as disease-causing agents, avian Haemosporida are of great interest [17]. Previous studies identified an enormous number of haemosporidian lineages worldwide from a great variety of bird species. Data from over 4704 unique genetic lineages from 2182 bird species have already been collected in the MalAvi database (as of July 2022; [18]). Madagascar seems to be a biodiversity hotspot for Plasmodium and Haemoproteus species [16].
Trypanosoma is the most abundant and important genus of kinetoplasts [19]. These protozoan blood parasites infect different vertebrate hosts, where they occupy the bloodstream or an intracellular environment, and are transmitted by a wide variety of blood-sucking arthropods. Avian trypanosomes are not vertebrate-host specific; rather, strains successfully develop in numerous species of distant avian hosts [20]. In contrast to their mammalian relatives, avian trypanosomes are, in most cases, harmless to their hosts and remain understudied [21]—there are only seven well-characterized species from birds so far for which molecular and morphological data are available [19]. Filarioid nematodes are highly specialized parasites of vertebrates with a worldwide distribution. Adults infect tissue and tissue spaces, whereas larval stages can be found in the peripheral blood as circulating microfilariae [22]. Different blood-sucking insects are intermediate hosts of parasitic nematodes and enable transmission [23]. Although microfilariae are quite easy to detect microscopically, identification is challenging due to the similarities in their morphology. Of the approximately 160 avian filarioid species described to date, DNA sequences of only six species are currently available, which makes molecular identification usually impossible [22].
This study provides new insights into the diversity of these blood parasites present in vangas and other endemic Corvoidea bird species on Madagascar. Molecular data on blood parasites, such as the Haemosporida, trypanosomes and microfilariae, provide evidence for inter- and intra-specific co-occurrences.
2. Materials and Methods
Blood samples were collected in the Maromizaha rainforest located in the eastern part of Madagascar (18°58′8″ S, 48°27′48″ E), 30 km from Moramanga city, at an altitude between 943–1213 m. Birds were caught with mist nets in the months September–January (2003–2007, 2010, 2012, 2014, 2016 and 2018), with the majority of samples taken in November and December, coinciding with the breeding season of many bird species in the study area [24]. A blood sample was taken by puncturing the brachial vein. The protocol was approved by the Direction de la Préservation de la Biodiversité, Antananarivo, Madagascar. Blood was immediately stored in lysis buffer [25], and, if possible, one blood smear per bird was prepared before the bird was released back into the wild. A total of 131 bird blood samples of different Corvoidea were collected: Coracina cinerea (Campechagidae, n = 4), Dicrurus forficatus (Dicruridae, n = 8), Terpsiphone mutata (Monarchidae, n = 40), and different species of the bird family Vangidae (n = 79).
Blood smears (n = 56) were prepared in the years 2007, 2012, 2014, 2016 and 2018, dried on site and fixed with 99% methanol for 10 min in the field. Using the Hemacolor® staining set (Merck KGaA, Darmstadt, Germany) the blood smears were stained with Giemsa following the manufacturer’s protocol. Every slide was examined using an AxioImager M2 (Carl Zeiss AG, Oberkochen, Germany). At first, slides were screened at 400× magnification for 10 min to quickly detect larger parasites (Leucocytozoon spp., trypanosomes, and filarioid nematodes) and then 20 min under high magnification (×100 oil-immersion objective, ×10 ocular) to detect smaller ones (Plasmodium and Haemoproteus spp.). Pictures were taken and edited with Zen software (Carl Zeiss AG, Oberkochen, Germany).
Total DNA extraction of blood samples was performed using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. DNA concentration and purity were quantified using a NanoDrop N50 UV-Vis spectrophotometer (Implen GmbH, Munich, Germany) and stored at −20 °C until further use.
Molecular parasite detection was performed using different PCR setups. In each test run a positive control as well as a negative control (nuclease-free water) was included.
Screening for haemosporidian parasites was performed using a multiplex PCR approach [26] with specific primer pairs for simultaneous DNA amplification of Plasmodium (PMF/PMR), Haemoproteus (HMF/HMR) and Leucocytozoon (LMF/LMR). The reactions were set up in a total volume of 10 µL containing 5 µL of 2× Multiplex PCR Master-Mix (Quiagen, Hilden, Germany), 0.2 µL of each primer (10 µM) and 3.8 µL of DNA template. If the DNA concentration was higher than 10 ng/µL, the DNA template was diluted with nuclease-free water. Cycling conditions were performed as described by Ciloglu et al. [26]. Amplification products (10 µL) of the multiplex PCR were mixed with GelRedTM stain (BIOTREND, Köln, Germany) and electrophoretically resolved after 45 min at 90 V in 2% agarose gels. The different parasite genera apparent in the samples were determined by identifying the size of the resulting amplification fragments (Plasmodium: 377–379 bp; Haemoproteus 525–533 bp; Leucocytozoon 218 bp).
For identification purposes, all samples were additionally screened using aa nested PCR targeting a 479 bp region of the cytochrome b gene (cytb) of the haemosporidian parasites [27,28]. PCR reactions of the first PCR were carried out in a total volume of 25 µL, containing 2.5 µL GeneAmp™ 10X PCR Buffer II (Applied Biosystems, Carlsbad, CA, USA), 2 µL MgCl2 (25 mM), 1 µL of each primer (HaemNF/HaemNR3; 10 mM), 0.5 µL of each dNTP (10 µmol), 0.125 µL AmpliTaq™ DNA polymerase (5 U/µL; Applied Biosystems, Carlsbad, CA, USA), 5 µL template DNA (10–100 ng/µL) and 12.875 µL nuclease-free water. The reaction mixture of the nested PCRs consisted of 5µL GeneAmp™ 10X PCR Buffer II (Applied Biosystems, Carlsbad, CA, USA), 4 µL MgCl2 (25 mM), 2 µL of each primer (HaemF/HaemR2 for Plasmodium/Haemoproteus detection and HaemFL/HaemR2L for Leucocytozoon detection; 10 mM), 1 µL of each dNTP (10 µmol), 0.25 µL AmpliTaq™ DNA polymerase (5 U/µL; Applied Biosystems, Carlsbad, CA, USA), 2 µL amplification product of the initial PCR and 33.75 µL nuclease-free water in a total volume of 50 µL. Cycling conditions of both PCRs were performed as described by Hellgren et al. [27]. Amplification products (5µL) of the nested PCR were also mixed with GelRedTM stain and then visualized on a 1.5% agarose gel after 20 min at 90 V.
Presence of Trypanosoma DNA was detected by nested PCR targeting a 770 bp SSU rRNA fragment [20]. The first set of primers was Tryp763 and Tryp1016 and the second set was Tryp99 and Tryp957. Reaction mixtures were equal to the nested PCR used for haemosporidian detection. Cycling conditions of both PCRs were performed as described by Valkiūnas et al. [20]. Amplification products (5 µL) of the nested PCR were also mixed with GelRedTM stain and then visualized on a 1.5% agarose gel after 20 min at 90 V.
The molecular detection of microfilariae was not possible using already published PCR protocols [29,30]. Probably due to low concentrations of DNA, one-step PCRs were not sufficiently sensitive. Using Geneious v. 2021.1.1 (https://www.geneious.com), primers for specific nested PCRs were developed. Screening for microfilariae was performed using a nested PCR assay targeting a 28S rRNA fragment. Using the primers 28SNemF1 (3′—GAGTGAAAAGGAAAAAGCCC—5′) and 28SNemR1 (3′—ATCCGTGTTTCAAGACGGG—5′) for the first PCR, an approximately 790 bp fragment could be amplified. The nested PCR amplified an approximately 690 bp fragment of 28S rRNA nested within the first fragment, using the primer pair 28SNemF2 (3′—ATGGAAATGTAGCGTATAGG—5′) and 28SNemR2 (3′—GGTGATTAACGTTCACATCG—5′). PCR reaction of the first PCR was carried out in a total volume of 25 µL, containing 2.5 µL GeneAmp™ 10X PCR Buffer II, 2 µL MgCl2 (25 mM), 1 µL of each primer (10 mM), 0.5 µL of each dNTP (10 µmol), 0.125 µL AmpliTaq™ DNA polymerase, 5 µL template DNA (10–100 ng/µL) and 12.875 µL nuclease-free water. The cycling profile consisted of denaturation at 94 °C for 5 min; followed by 94 °C/30 s, 51 °C/ 30 s, 72 °C/45 s for 25 cycles; and a final extension at 72 °C for 7 min. The reaction mixture of the nested PCR consisted of 5 µL GeneAmp™ 10X PCR Buffer II, 4 µL MgCl2 (25 mM), 2 µL of each primer (10 mM), 1µL of each dNTP (10 µmol), 0.25 µL AmpliTaq™ DNA polymerase, 2 µL amplification product of the initial PCR and 33.75 µL nuclease-free water in a total volume of 50 µL. The cycling profile consisted of denaturation at 94 °C for 5 min; followed by94 °C/30 s, 49 °C/ 30 s, 72 °C/45 s for 35 cycles; and a final extension at 72 °C for 7 min. Amplification products (5 µL) were mixed with GelRedTM stain and then visualized on a 1.5% agarose gel after 20 min at 90 V.
A second nested PCR, targeting a fragment of the cox1 gene, was developed and used for samples where DNA amplification of the microfilarial 28S rRNA fragment was successful. Using the primers COINemF1 (3′—TATAATTCTGTTCTTACTATGCATGG—5′) and COINemR1 (3′—GGAATAGCAATAATGATAGTAGCAGC—5′) for the first PCR, an approximately 730 bp fragment was amplified. The nested PCR amplified an approximately 650 bp fragment of cox1 nested within the first fragment, using the primer pair COINemF2 (3′—CCTATTTTGATTGGTGGTTTTGG—5′) and COINemR2 (3′—CTAAAATAAGTACGAGTATCAATATC—5′). PCR reactions were carried out as for 28S rRNA-nested PCR. The cycling profile of the first PCR consisted of denaturation at 94 °C for 5 min; followed by94 °C/30 s, 55 °C/ 30 s, 72 °C/45 s for 25 cycles; and final extension at 72 °C for 7 min, while the profile of the nested PCR differed in an annealing temperature at 51 °C and a total of 35 cycles. Amplification products (5 µL) were mixed with GelRedTM stain and then visualized on a 1.5% agarose gel after 20 min at 90 V.
All amplification products were purified using the PCR Product Purification Kit (Roche, Mannheim, Germany). After sequencing (Microsynth AG, Balgach, Switzerland), the resulting sequence data were checked and edited using Geneious v. 2021.1.1 (https://www.geneious.com). The final sequences were then distinguished by identifying their closest matches in GenBank [31] using the NCBI nucleotide BLAST search. Avian haemosporidian parasites were additionally identified using the BLAST search of the MalAvi database [18]. If sequences differed in at least one basepair, it was considered a new lineage and naming was performed according to the system used for the MalAvi database: the first three letters of the bird genus, the first three letters of the species name, followed by a number (e.g., XENPOL01 from Xenopirostris polleni). In the text, haemosporidian lineage names were always accompanied by an abbreviation of its parasite genus (p: Plasmodium, h: Haemoproteus, l: Leucocytozoon). Newly amplified sequences of trypanosomes and filarioid nematodes were named according to their closest match in GenBank and given an isolate abbreviation for easier identification. All newly detected parasite lineages/sequences were deposited in GenBank (accession numbers OP006583-OP006613; Table S2 in Supplementary Materials).
Phylogenetic analyses of haemosporidian lineages, trypanosomes and filarioid nematodes detected in this study were performed using MEGA v.10.2 [32]. The dataset used for the first phylogenetic reconstruction consisted of cytochrome b sequences of haemosporidian lineages obtained in this study and reference sequences of different morphospecies of Haemosporida downloaded from GenBank, each trimmed to 462 bp to ensure consistency in sequence length. A cytochrome b sequence from Theileria annulata (KF732030) was used as an outgroup. The second tree included all 18S rRNA sequences of Trypanosoma lineages detected in the sample set and sequences of homologous Trypanosoma sequences from GenBank, each trimmed to 745 bp. The human pathogen Trypanosoma brucei (AF306777) was used as an outgroup. The third dataset consisted of all sequences of microfilariae detected in this study. Partial sequences of cox1 and 28S rRNA were concatenated to perform phylogenetic analyses with a fragment of 1175–1342 bp length. Ascaridia galli (KY990014 and KT613888) was used as an outgroup. Phylogenies were generated by implementing the best-fitting model, which was identified by MEGA v.10.2 for every parasite taxon. All maximum likelihood methods were performed using 1000 replicates. The resulting phylograms were viewed and edited with MEGA v.10.2 [32].
3. Results
The molecular screening (multiplex and nested PCR) of 131 blood samples of different Corvoidea species allowed the detection of haemosporidian infections in 71 samples (54.2%) and Trypanosoma infection in 28 samples (21.4%). DNA of filarioid nematodes was detected in seven samples (5.3%) (Table 1). In 14 of the 56 blood smears obtained, gametocytes of Haemosporida were detected. Just one blood smear contained microfilariae (1.8%), whereas two blood smears revealed trypanosomes (3.6%). The vangas showed the highest prevalence for haemosporidian parasites with 79.7%, whereas most of the Trypanosoma infections (27.5%) were detected in samples of Terpsiphone mutata (Monarchidae). Leucocytozoon was the only parasite taxon detected in blood samples of the ashy cuckoo shrike (Campephagidae), whereas samples of the Dicrurus forficatus (Dicruridae) showed only one infection with Trypanosoma. All kinds of parasite taxa (Haemosporida, Trypanosoma and microfilariae) were detected in the Terpsiphone mutata, Newtonia amphicroa and Tylas eduardi samples, although Haemoproteus infections were absent in Terpsiphone mutata samples.

Table 1.
Dataset of Corvoidea species examined in this study. Absolute numbers of blood smears (Nbs) and blood samples (N) are given. Results of parasite detections are listed for haemosporidian parasites, trypanosomes and filarioid nematodes, respectively. For each parasite taxon, percentages (%) of infected samples identified with microscopic method (Pmic) and molecular methods (Pmol), along with the name of the identified lineage/sequence, are given. “-” indicates that percentages were not measurable due to a lack of samples or that no sequence was amplified. If the parasite lineage/sequence was detected more than once, sample sizes are given in brackets. Newly detected lineages/sequences are given in bold letters.
3.1. Microscopic, Molecular, and Phylogenetic Analyses of Haemosporidian Parasites
A total of 44 haemosporidian lineages (seven Plasmodium, 15 Haemoproteus and 22 Leucocytozoon spp.) were detected in the blood samples of birds. The majority of Coracina cinerea samples (n = 3) contained exclusively DNA of Leucocytozoon lineage lFOUOMI07. Gametocytes of this parasite were microscopically detected in blood smears (Figure 1k).
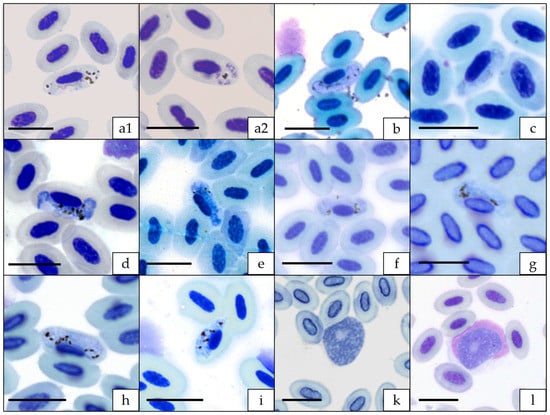
Figure 1.
Blood stages of haemosporidian parasites of Corvoidea species from Madagascar. (a1) macrogametocyte and (a2) erythrocytic meront of Plasmodium pCOPALB03 from Terpsiphone mutata; (b): Haemoproteus hLEPCHA01 from Leptopterus chabert; (c): Haemoproteus hNEWAM04 from Newtonia amphicroa; (d): Haemoproteus hNEWBR03 from Newtonia brunneicauda; (e): Haemoproteus hNEWBR04 from Newtonia brunneicauda; (f): Haemoproteus hNEWBR05 from Newtonia brunneicauda; (g): Haemoproteus hNEWBR07 from Newtonia brunneicauda; (h): Haemoproteus hPSEWAR01 from Pseudobias wardi; (i): Haemoproteus hVANCUR01 from Vanga curvirostris; (k): Leucocytozoon lFOUOMI07 from Coracina cinerea; (l): Leucocytozoon lVANCUR02 from Vanga curvirostris. Blood smears were fixed with absolute methanol and stained with Giemsa. Scale bar = 10 µm.
All samples of Dicrurus forficatus were negative for haemosporidian infection (Table 1). Five samples (12.5%) of Terpsiphone mutata contained DNA of Plasmodium sp. (pCOPALB03; n = 2) or Leucocytozoon spp. (lCINSOV02, lTERMUT01 and one unidentified mixed infection). Gametocytes and meronts were microscopically detected in one blood smear referring to a blood sample containing DNA of pCOPALB03 (Figure 1(a1,a2)). Leucocytozoon infections were missed using only multiplex PCR (See Table S1 in Supplementary Materials). Vangas showed the highest diversity of haemosporidian parasites and high prevalences for all three genera, with 49.4% Plasmodium, 45.6% Haemoproteus and 51.9% Leucocytozoon, respectively (Figure 2). Most of the infected samples contained mixed infections (60.4%), with 41.3% double infections, 14.3% triple infections and 4.8% quadruple infections. All combinations of mixed infections (P/H; P/L; H/L) occurred with similar frequencies. Gametocytes of eight Haemoproteus and two Leucocytozoon lineages were microscopically detected (Figure 1b–l).
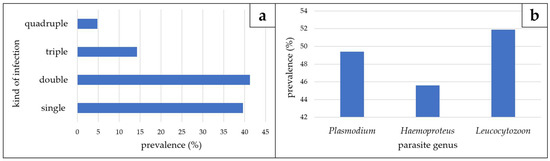
Figure 2.
Kind of infection (a) and parasite prevalence (b) detected in species of the Vangidae examined throughout this study (n = 79).
The majority of haemosporidian lineages (n = 40), of which 15 were detected for the first time in this study, were isolated from vangas (Table 2). A high amount of the Newtonia amphicroa samples (34.6%) were found to be infected with three different Plasmodium lineages (pNEWAM05, pNEWAM01 and pNEWAM07), the highest diversity of Plasmodium lineages detected in our examined bird species. For Haemoproteus lineages, Newtonia brunneicauda showed the highest diversity, with six different lineages (hNEWBR01-05 and hNEWBR07) and a high prevalence for Haemoproteus (75%). Calicalicus madagascariensis was infected with six lineages of Leucocytozoon (lANLAT11, lCALMAD01-04 and lHYPMA02), thereby showing the highest diversity, with a prevalence of 58.3%. Just a few of the haemosporidian lineages were shared by different vanga species (Table 2). It is noteworthy that the vast majority of lineages detected throughout this study have been found exclusively on Madagascar. Plasmodium relictum (pGRW04) is the only lineage detected worldwide, whereas Haemoproteus micronuclearis hRBQ11 and Leucocytozoon lineage lANLAT11 have also been detected in African birds. lFOMAD01 has been detected in birds on Madagascar, as well as in various Foudia species (Ploceidae) on Mayotte and Comoros, and in Hypsipetes madagascariensis (Pycnonotidae) from Madagascar.

Table 2.
Haemosporidian lineages found in our study. Isolation source is given as bird species name and total number of detections within the species (N). Furthermore, the percentage (%) of the lineage in the absolute infected samples of the bird species with regard to the parasite genus is also listed. Data of other bird hosts and sites of those hosts were drawn from the MalAvi database (as of July 2022). “-” indicates that no other host has been reported. Bold text indicates lineages for which gametocytes were detected in blood smears.
All haemosporidian genera appeared as monophyletic groups (Figure 3). Within the Plasmodium subtree pNEWAM05 and pCOPALB03 formed an independent clade. They differed in 18 bp on the 662 bp fragment of cytochrome b which was used for phylogenetic analysis. pNEWAM07 and pBERZOS01 formed another clade. These lineages differed in only two bp but were isolated from completely different bird species. pFOUMAD03 differed in only one single basepair from Plasmodium relictum pGRW04, whereas pNEWAM01 showed no close relationship to the other lineages detected in the Corvoidea samples.
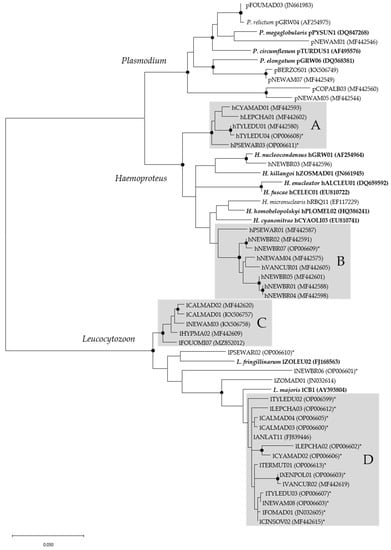
Figure 3.
Phylogenetic relationship of 56 mitochondrial cytb lineages of haemosporidian parasites detected in blood samples of birds belonging to the superfamily Corvoidea on Madagascar, along with sequences of different morphospecies, constructed using maximum likelihood (GTR + G). Dots on nodes indicate bootstrap values >70%. In brackets are given GenBank accession numbers for all lineages. Morphospecies are written where linked to the characteristic lineages. Those in bold were not detected throughout this study. New lineages that were found for the first time in the present study are marked with an asterisk (*). Grey boxes (A–D) indicate groups of closely related cytb lineages.
3.2. Microscopic, Molecular and Phylogenetic Analyses of Trypanosomes
Within the Haemoproteus subtree, the lineages detected throughout this study mainly appeared in two distinct clades (Figure 3A,B). The lineage hNEWBR03 showed the greatest homology with H. nucleocondensus hGRW01 with eight basepair differences. Haemoproteus micronuclearis hRBQ11, which was detected once in the sample set, was not closely related to the other lineages of Haemoprotues isolated from Vangidae in this study.
The majority of Leucocytozoon lineages detected in this study also formed two separate clades (Figure 3C,D). Clade D showed a close relationship to the morphospecies Leucocytozoon majoris lCB1. The lineages lPSEWAR02, lNEWBR06 and lZOMAD01 showed no close relationship to other lineages included in this analysis.
The highest prevalence for Trypanosoma infections was found in the family Monarchidae (Terpsiphone mutata) (27.5%), followed by vangas (20.3%), with a single confirmation of Trypanosoma DNA in Dicrurus forficatus (12.5%). Two microscopic detections of trypanosomes were made in two samples of Vangidae: one in Leptopterus chabert and one in Vanga curvirostris. However, the trypanosome in the L. chabert could not be determined more precisely by molecular methods because amplification of the gene fragment failed. The parasite in V. curvirostris was identified as the newly described T. avium isolate CORVOID4 (OP006598; Figure 4).
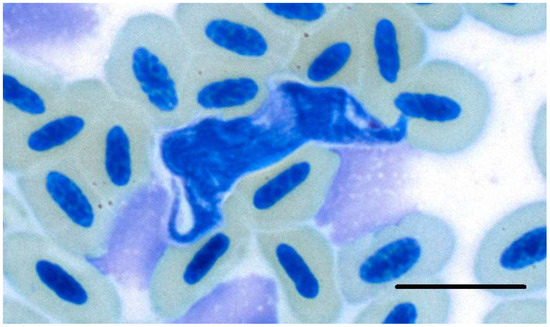
Figure 4.
Trypomastigote form of Trypanosoma avium isolate CORVOID4 (OP006598) detected in a blood smear of Vanga curvirostris in Madagascar. Scale bar = 10 µm.
Overall, 10 different Trypanosoma sequences of the SSU rRNA were detected in the Malagasy Corvoidea samples (Table 1). The sequences were assigned to three known species (T. avium, T. anguiformis and T. lewisi); two sequences still need to be identified to species level (T. sp. CORVOID01 and 02). Three sequences were 100% homologous to previously published sequences (T. avium (KT728401 and KT728402) and T. lewisi (GU252209)). Five of the detected Trypanosoma sequences were shared between different bird genera. Trypanosoma avium (KT728402) was the most abundant detected sequence, isolated from five Terpsiphone mutata and four vangas. The highest sequence richness in a single bird species was found in T. mutata, whereas the highest prevalence (50%) was found in Pseudobias wardi (n = 2) and V. curvirostris (n = 1). Exceptionally low prevalence (7.7%) was detected in Newtonia amphicroa (n = 26). The only sequence detected in Dicrurus forficatus was the T. avium isolate CORVOID2, a sequence additionally found once in Newtonia brunneicauda.
Phylogenetically, all sequences formed a monophyletic branch (Figure 5), except for the sequence homologous to T. lewisi (GU252209), which formed another branch with the complete homologous sequences of T. blanchardi (AY491764), T. musculi (AJ223568) and T. niviventerae (AB242274). These sequences have previously been detected exclusively in rodents and are described as T. lewisi-like trypanosomes [33]. Sequences forming the other branch have to date been exclusively detected in bird hosts. This branch was again divided into two branches. One contained sequences assigned to the species T. avium, the other contained sequences related to T. anguiformis or an unknown species. Although the Trypanosoma sp. isolate CORVOID01 forms a clade with sequences of T. anguiformis, it is considered as an unknown species as this sequence differs in at least eight basepairs.
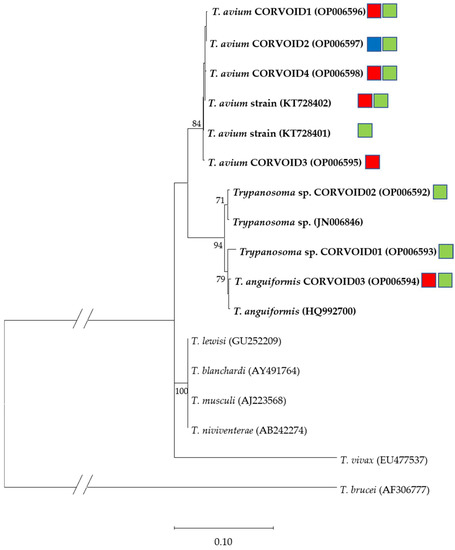
Figure 5.
Phylogenetic relationship of Trypanosoma SSU rRNA sequences (Acc. No.) detected in Corvoidea species on Madagascar, along with highly homologous previously published sequences (Acc. No.) constructed using maximum likelihood (K2 + G). Bootstrap values >70% are given. Bold text indicates sequences exclusively isolated from birds. Bird families from which the lineages were isolated are indicated with color-coded squares. Red = Monarchidae; blue = Dicruridae; green = Vangidae.
3.3. Microscopic, Molecular, and Phylogenetic Analyses of Microfilariae
The amount of filarioid nematode infections was extremely low (5.3%). The highest prevalence was found in Mystacornis crossleyi (Vangidae), with three positive samples (50%; Table 1). Morphological detection was possible for the Onchocercidae sp. isolate DANEW in a blood smear of Newtonia amphicroa (Figure 6). One blood sample of the latter contained a mixed infection, identified by numerous double peaks in the chromatogram of the 28S rRNA sequence. The cox1 sequence of this sample was identified as Onchocercidae sp. isolate DANEW.
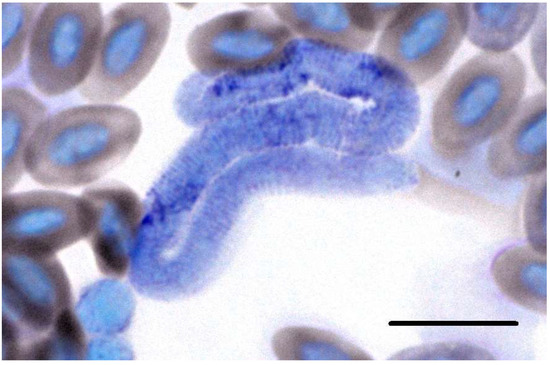
Figure 6.
Larval stage (microfilaria) of Onchocercidae sp. isolate DANEW (OP006587 and OP006583) isolated from Newtonia amphicroa (Vangidae) in Madagascar. Scale bar = 10 µm.
Four different 28S rRNA sequences were identified linked to five cox1 sequences. The 28S rRNA of Onchocercidae sp. isolate CROSSBAB was found in three M. crossleyi samples, but two different cox1 sequences were isolated from them (CROSSBAB and CROSSBAB2) with two basepair changes that distinguished these sequences. No sequence was shared between different bird species or families. No sequence was 100% homologous to previously published sequences.
Phylogenetic analysis (Figure 7) revealed close relatedness of the isolate from Tylas eduardi to Splendidofilaria bartletti, a nematode detected in a single Sylvia atricapilla from Lithuania [22]. The Onchocercidae sp. isolates CROSSBAB and CROSSBAB2 were quite homologous, with just two basepair changes and formed an individual branch within the tree. All microfilariae isolated from Vangidae appeared in the same clade, whereas the isolate from Terpsiphone mutata (Monarchidae), Aproctella alesandroi isolate PARFLY was separated from them.
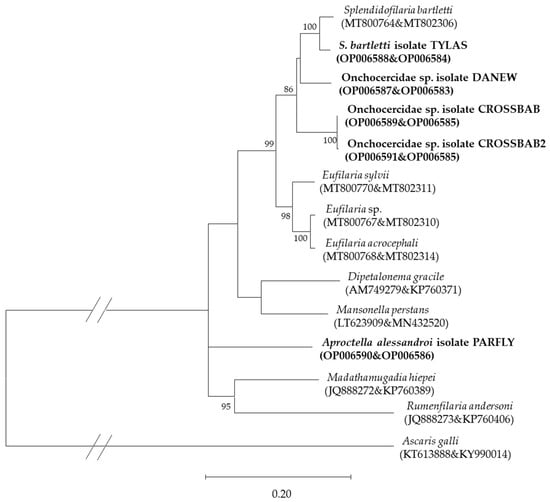
Figure 7.
Phylogenetic relationship of concatenated filarioid nematode cox1 and 28S rRNA sequences (Acc. No.) detected in Corvoidea species on Madagascar, along with previously published sequences of avian filarioid nematodes, constructed using maximum likelihood (GTR + G + I). Bold text indicates sequences isolated in this study. Bootstrap values >70% are given.
4. Discussion
The parasite abundance and diversity detected in the Corvoidea species on Madagascar demonstrate how blood parasite infections can vary among closely related bird families or species.
4.1. Haemosporida
The prevalence of haemosporidian parasites varied greatly between the closely related Corvoidea species. Whereas vangas harbored an enormous diversity and high abundance of Haemosporida, the other examined families showed little prevalence of haemosporidian infections. The only exception was Coracina cinerea (Campephagidae), which had no infection with Plasmodium and Haemoproteus spp. but a high prevalence of Leucocytozoon spp. Three of the four blood samples examined contained DNA of Leucocytozoon lineage lFOUOMI07. This lineage has previously been found in one blood sample of Foudia omissa (Ploceidae) on Madagascar [34]. In our study, gametocytes were detected in blood smears of C. cinerea, which confirms that this bird species is a suitable host for lFOUOMI07. Since DNA of lFOUOMI07 was detected in only one of the 207 Foudia blood samples, and no gametocytes were found by Musa et al. [34], it is assumed that this single case might represent an abortive infection and that lFOUOMI07 is specialized on Coracina cinerea. Barraclough et al. [14] detected Leucocytozoon coracinae in Coracina cinerea blood smears. They described the parasite as a ‘typically small round morph’ that presumably occurs in the different species of Campephagidae [14]. We were not able to compare the gametocytes of lFOUOMI07 with those of L. coracinae as our detected gametocyte was not connected with the nucleus of the host cell, which is a prerequisite for the identification of the host species. However, it is possible that lFOUOMI07 does represent the cytb sequence of L. coracinae.
For Dicrurus forficatus, there were conflicting results with respect to Haemosporida prevalence. In our study, no haemosporidian lineage was detected (n = 8). However, Savage et al. [11,35] found gametocytes of Haemoproteus dicruri and H. khani in blood smears of 11 birds (n = 18). This difference might have been due to the absence of suitable vectors in the Maromizaha rainforest, or the low number of samples examined in this study. More data is needed to test these hypotheses.
Low haemosporidian prevalence in Terpsiphone mutata (Monarchidae) was not only found in our study (12.5%) but has also been reported in several other studies [11,12,15]. Greiner et al. [13] detected Plasmodium spp. in a single blood smear of T. mutata. However, no further information about absolute sample size or the morphology of the gametocytes or erythrocytic meronts are given. Twenty blood smears of Monarchidae species, presumably T. mutata, were examined by Raharimanga et al. [12]. In three blood smears (15%) gametocytes of Plasmodium or Haemoproteus species were detected but no further determination was provided. Savage et al. [11] examined a total of 90 blood smears of T. mutata for the presence of haematozoan parasites. They detected Haemoproteus species in three of the examined samples (3.33%). Again, further information about parasite species identity or morphology was missing. Ivanova et al. [15] examined nine T. mutata using both morphological and molecular methods. Neither method provided evidence of haemosporidian infection. In our study, we detected the Plasmodium lineage pCOPALB03 in two samples (5%) and two different Leucocytozoon lineages, as well as an unidentified mixed infection in three other samples. In contrast to Savage et al. [11], we did not detect any Haemoproteus. However, we were able to identify gametocytes, as well as erythrocytic meronts of pCOPALB03, and, therefore, demonstrated that T. mutata is a suitable host of this lineage. The species description of this parasite is still pending. The lineage itself has previously been detected in only a single blood sample of Copsychus albospecularis (Muscicapidae) on Madagascar [16]. Further studies are needed to rule out whether this parasite might be specialized on T. mutata and if it is restricted to Madagascar.
Considering a total of 11 species, we examined half of the existing Vangidae species. In contrast to the Corvoidea families, vangas showed high prevalence for haemosporidian parasites (79.7%). This is in accordance with other studies that examined blood samples or blood smears of vangas [11,12,15,16]. Vangas harbored an enormous diversity of avian haemosporidian parasites, with six Plasmodium, 15 Haemoproteus and 18 distinct Leucocytozoon lineages detected in our sample set. The parasites found in this family seem, in most cases, to be highly specialized. Haemoproteus species are known, in general, to be highly specialized, whereas Plasmodium species tend more often to be generalists [16]. For Leucocytozoon species, very little information exists about specialization but there seem to be both highly specialized species and generalists [16,36,37]. Detailed examples of highly specialized lineages from our study are provided below.
Cyanolanius madagascarinus, for example, was found to be infected with hCYAMAD01 and lCYAMAD02. Both lineages were found exclusively in this bird species (Table 2). We assume that both lineages are specialized on this bird species and restricted to Madagascar. Savage et al. [11] examined a single blood smear of C. madagascarinus and detected gametocytes of Haemoproteus vangii and Leucocytozoon lairdi. These are probably morphospecies of the lineages we detected, but, as we did not have blood smears for morphological examination, we were not able to compare gametocytes.
Mystacornis crossleyi is a vanga species which showed low haemosporidian prevalence. We detected Haemoproteus micronuclearis (hRBQ11) in one blood sample (16.6%). This species is reported to be specialized on birds of the family Ploceidae [16]. Since such an infection in M. crossleyi seems to be very unlikely, it could be an abortive infection, which would mean that M. crossleyi is not a natural host. However, we had no blood samples to resolve this uncertainty. Savage et al. [11] examined two blood smears of M. crossleyi but did not detect gametocytes of Haemosporida. Ivanova et al. [15] detected the generalist Leucocytozoon lineage lFOMAD01 in a single sample of M. crossleyi. However, they were not able to detect gametocytes of this lineage in the blood smear of the bird. Thus, an abortive infection cannot be ruled out, or M. crossleyi was just an accidental host.
Newtonia amphicroa was the vanga with the highest number of blood samples in our study. Compared to the other Vangidae species, we detected a high diversity regarding the genus Plasmodium. pNEWAM05 seems to be a generalist restricted to Madagascar as this lineage was also isolated from bird species of other avian families [16]. However, pNEWAM07 might be specialized on Vangidae species, whereas pNEWAM01 might be specialized on Newtonia species. All lineages have been exclusively detected on Madagascar (Table 2). The Haemoproteus linage NEWAM04 was the only one detected in N. amphicroa in this study. Gametocytes of this lineage were also found in blood smears (Figure 1c). Savage et al. [11] detected gametocytes of H. vangii in two of 18 blood smears of N. amphicroa. The comparison of the gametocytes of H. vangii and hNEWAM04 revealed many similarities, including fine pigment granules. However, the quality of our blood smears was not sufficiently good to compare all measurements and perform morphological determination to species level. We still predict that hNEWAM04 will represent the cytb sequence of H. vangii. It has been suggested that H. vangii has additional Vangidae species as hosts [11]. As hNEWAM04 seems to be specialized on N. amphicroa, other gametocytes found might be either cryptic species with similar morphology or H. vangii has different haplotypes which show specialization on different vanga species. Further studies are needed to resolve this question.
Just two samples of N. amphicroa (7.7%) contained DNA of Leucocytozoon species. One lineage, lFOMAD01, has recently been reported to be a highly abundant generalist on Madagascar [34]. However, this was the only finding of lFOMAD01 in our study. It is possible that, in this case, there is also an abortive infection and that the Corvoidea are basically resistant to this parasite. In contrast to our findings, Ivanova et al. [15] also detected the lineage hNEWAM02 in samples of N. amphicroa, as well as in N. brunneicauda samples and lNEWAM03 in two of three N. amphicroa samples. hNEWAM02 was not detected in any sample of our study, whereas lNEWAM03 was frequently detected in N. brunneicauda samples (41.7%) but not in N. amphicroa (n = 26). lNEWAM03 has additionally been detected only in a single Foudia omissa sample on Madagascar [16] to date. Due to the limited data in that study, the lineage was grouped with other Leucocytozoon lineages, and it was predicted that these might represent generalist species. Based on the newly available data from the present study, the most likely hypothesis is that the Leucocytozoon lineages of Vangidae, including lNEWAM03, are specialized and may even represent distinct species. With respect to this assumption, the finding of lNEWAM03 in Foudia omissa might, therefore, represent an abortive infection and, since N. amphicroa is hard to distinguish from N. brunneicauda, the results obtained by Ivanova et al. [15] should also be revised to corroborate their validity.
Newtonia brunneicauda was found to be infected with another, probably specialized, Leucocytozoon lineage—lNEWBR06. However, there was just a single detection of that lineage and, therefore, no reliable statement can be made. The diversity of Plasmodium and Leucocytozoon lineages in N. brunneicauda was rather low, especially in contrast to the Haemoproteus lineages found. Six lineages were detected in the 12 blood samples of N. brunneicauda in this study with hNEWBR05 being the most abundant lineage. Gametocytes of hNEWBR03-5 and hNEWBR07 were additionally detected in blood smears which confirms that N. brunneicauda is a competent host for those parasites. According to the phylogenetic analyses the six Haemoproteus lineages might be assigned to at least three different species, one consisting of hNEWBR03, one of the haplotypes hNEWBR02 and 07, and a third comprising the lineages hNEWBR01, 04 and 05 (Figure 3 and Table S3 in Supplementary Materials). However, comparison of macrogametocytes revealed morphological differences between hNEWBR04 and 05. hNEWBR01 and 04 may represent a separate species as well as hNEWBR05, which seems to be another specialized Haemoproteus species of N. brunneicauda. Further species determinations are needed to confirm this hypothesis.
Tylas eduardi samples harbored all three haemosporidian genera. Plasmodium relictum (pGRW04) was found in 33.3% of the samples. With hTYLED01/04 and lTYLEDU02/03, T. eduardi probably contained four specialized parasite lineages. The lineage lCALMAD01, found twice in T.eduardi, was additionally identified in Calicalicus madagascariensis. It was the only Leucocytozoon lineage shared between Vangidae species. As findings of this lineage have been restricted to Vangidae [15], possible specialization at bird family level is still predicted. Savage et al. [11] described Leucocytozoon lairdi from various Vangidae species, including T. eduardi. It can be assumed that lCALMAD01 might be the cytochrome b sequence of L. lairdi. Because no gametocytes of lCALMAD01 were found in this study, this hypothesis cannot be verified.
Calicalicus madagascariensis harbored the highest diversity of Leucocytozoon lineages of all the examined bird species. Besides lCALMAD01 there were three other lineages (lCALMAD02-04) that might be specialized on C. madagascariensis. Phylogenetic analyses revealed a remarkably close relationship of lCALMAD01 and 02, as well as lCALMAD03 and 04. Due to low genetic difference (2 bp), lCALMAD03 and lCALMAD04 might be haplotypes of one species. Another Leucocytozoon lineage detected in C. madagascariensis was lHYPMA02. This lineage might be a highly abundant generalist in Madagascar [36], like lFOMAD01. However, as this was the only finding of lHYPMA02 in our study, resistance of all Corvoidea species against this parasite cannot be ruled out.
Vanga curvirostris is a suitable host for hVANCUR01 and lVANCUR02 as we detected gametocytes of both lineages in blood smears of this bird species. hVANCUR01 might be the cytb sequence of Haemoproteus madagascariensis, a species described and found exclusively in V. curvirostris by Savage et al. [38], as the morphology of the gametocytes is similar. In contrast to the assumption of Savage et al. [38] that this parasite will be found in the different vanga species on Madagascar, we predict specialization of this parasite species on V. curvirostris. Due to morphological similarities, we also assume that the Leucocytozoon lineage lVANCUR02 might represent L. benetti. This species was described by Savage et al. [38] and was detected in V. curvirostris and the helmet vanga, Euryceros prevostii [38]. This parasite might also be specialized on vangas. However, as Leucocytozoon species often show extensive cryptic diversity [37], it cannot be ruled out that lVANCUR02 represents a separate species specialized on V. curvirostris.
The only Plasmodium lineage detected in V. curvirostris samples in this study was pNEWAM07. This lineage was detected in several vanga species and is, therefore, assumed to be specialized on this bird family. Savage et al. [39] detected a low prevalence of P. parvulum at (0.3%) in three vanga species, including V. curvirostris. pNEWAM07 might be the matching cytb sequence for P. parvulum. However, as we were not able to find gametocytes or erythrocytic meronts in our samples, this hypothesis still needs to be verified in further studies.
Other examples of potentially specialized haemosporidian lineages were found in Leptopterus chabert (hLEPCHA01, lLEPCHA02 and lLEPCHA03), Pseudobias wardi (lPSEWAR02 and hPSEWA01/03) and Xenopirostris polleni (lXENPOL01). Further data are needed until reliable conclusions can be drawn.
4.2. Trypanosoma
Based on the assumption that Trypanosoma species are host-specific, at least 96 trypanosome species were formerly identified in birds, but this theory was rejected, as trypanosomes can be transmitted to multiple host bird species [40]. However, due to the pleiomorphic character of this taxon, an accurate morphological description of Trypanosoma species is extremely difficult and studies on this topic are problematic [40]. Sehgal et al. [40] provided the first molecular-based evidence that a single morphospecies of T. avium can infect several avian species, consistent with the idea of low host-specificity for Trypanosoma. T. avium is described as a single species, although it has been identified in Africa, north America, and Europe, and even though the multiple sub-species differed in terms of morphological criteria [41]. Morphological studies showed that T. avium parasitizes at least 13 passeriform bird species in sub-Saharan Africa [41] and many bird hosts belonging to the Nectariniidae family showed a high prevalence [40]. The predicted low host-specificity could be confirmed for the four haplotypes of T. avium identified in this study, of which the most abundant sequence, T. avium (KT728402), was isolated from five Terpsiphone mutata samples and four Vangidae samples. Savage et al. [11] also detected T. avium in three vanga species and Atelornis pittoides (Brachypteraciide) on Madagascar.
Currently, Trypanosoma anguiformis has only been reported in Ghana where it was found in two different areas, with an overall prevalence of six out of 104 infected bird species (5.8%) [20]. We found haplotypes of this species twice in Pseudobias wardi (Vangidae) and once in Terpsiphone mutata (Monarchidae), which are both endemic to Madagascar. Vectors for Trypanosoma species are louse flies (Hippoboscidae) and biting midges (Ceratopogonidae) [42,43]. T. anguiformis may have reached Madagascar from mainland Africa via infected vectors or bird hosts and haplotypes infecting the Malagasy avian fauna may have been developed.
The single infection of Tylas eduardi with Trypanosoma lewisi is surprising. T. lewisi belongs to a complex of obligatory rodent parasites found worldwide [44]. Trypanosoma can be cyclically transmitted via peroral contamination by flea feces or accidental ingestion of fleas [45]. As T. eduardi is primarily insectivorous [2], an infection could have occurred due to an ingestion of fleas. Even though T. lewisi trypanosomes are considered highly host specific, there have been studies of T. lewisi or T. lewisi-like infecting other species, such as monkeys [44], although these interspecies cross-infections rarely occur naturally [45]. We assume that this Tylas eduardi individual was probably weakened by its Plasmodium and Leucocytozoon co-infection and developed this unusual infection. However, mechanical transmission and abortive infection cannot be ruled out.
Sehgal et al. [40] described the possibility of hosting more than one Trypanosoma species in one bird species; however, this could not be confirmed in this study.
For the birds for which blood smears as well as blood samples were available, molecular methods were overall more sensitive than microscopic methods in the detection of trypanosomes. This might be explained by the fact that trypanosomes are usually quite rare in peripheral blood vessels [46].
4.3. Microfilariae
Seven bird blood samples contained DNA of filarioid nematodes (5.3%). These sequences could be assigned to five different specimens. Other studies have found DNA of Cardiofilaria and Eufilaria in Campephagidae, whilst DNA of Chandlerella was detected in Dicruridae but not in vangas [47]. In our study, we detected four DNA sequences in five samples of vanga species: Onchocercidae sp. isolate CROSSBAB/2, Splendidofilaria bartletti isolate TYLAS and Onchocercidae sp. isolate DANEW. A DNA sequence of the Aproctella alessandroi isolate PARFLY was found once in Terpsiphone mutata (Monarchidae). The results indicate that the nematodes are very host specific. Savage et al. [11] also found microfilariae in blood smears of Vangas, but more data about the species identity was not provided. In contrast to other studies [11,47], we did not detect any filarioid nematodes in samples of Dicrurus forficatus. However, the small dataset (n = 8) limited the possibility of detection.
For the other three species mentioned in the results, little information is available, not only on the possible transmitting vectors, but also on the first classification of the species and, therefore, their host type.
5. Conclusions
Corvoidea on Madagascar showed extreme differences in their parasite abundance, composition, and diversity. Coracina cinerea (Campephagidae), Dicrurus forficatus (Dicruridae) and Terpsiphone mutata (Monarchidae) showed a low susceptibility for avian haemosporidian parasites. However, the sample sizes for Coracina cinerea and Dicrurus forficatus were too small to enable final conclusions to be drawn. Except for single specialized parasites, such as pCOPLAB03 and lFOUOMI07, these members of the Corvoidea seem to be quite resistant to haemosporidian parasites. In contrast to potential resistance against Haemosorida, Terpsiphone mutata showed a high prevalence and diversity of Trypanosoma species. Vangas, in general, seemed to be more susceptible to all kinds of blood parasites as they harbored a variety of so-called host-specific haemosporidian parasites of all genera with high prevalence. Whereas trypanosomes seem to be generalists, the filarioid nematodes detected might be specialized. These findings offer interesting insights into the complex host-parasite interactions that have evolved in each family or species of the Corvoidea on Madagascar. Future studies on Madagascar with a larger dataset, including more species from the different families referred to in this study, are needed to test the possibility of co-existence between blood parasites or the specificity of the parasite-host and host-vector relationships.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14100888/s1, Table S1: Haemosporidian detections in blood samples of Corvoidea species from Madagascar (n) using multiplex and nested PCR.; Table S2: Newly described lineages/sequences in our study. Accession number, target gene fragment and names are given.; Table S3: Genetic distances between Haemoproteus lineages isolated from Newtonia brunneicauda (Vangidae) on Madagascar based on a 462 bp fragment of cytochrome b.
Author Contributions
S.M. conceived of and designed this study; F.W. collected the samples in the field; R.M.V. and S.M. performed the molecular analyses; S.M. performed the morphological analyses; R.M.V. and S.M. performed the data analyses; R.M.V. and S.M. prepared the manuscript; F.W., A.D. and U.M. wrote the review and undertook editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), grant number 457213393.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are indebted to the Malagasy authorities for granting all the relevant research and export permits and to the Groupe d’Étude et de Recherche sur les Primates de Madagascar (GERP), namely Jonah Ratsimbazafy and Rose Marie Randrianarison, for letting us work at Maromizaha. Hajanirina Rakotomanana, and Daniel Rakotondravony at the Department of Animal Biology, University of Antananarivo supported us throughout. We thank Jean-Robert Lekamisi, Lova Tahiry Rasolondraibe, Onja Randriamalala, Nicola Lillich, Pia Reufsteck, Jean-Louis Berthoud and all the other Malagasy and German assistants for their help in the field. Finally, we want to thank three anonymous reviewers for their constructive comments on our draft manuscript that helped to improve it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, F.; Safford, R.; Skerrett, A. Birds of Madagascar and the Indian Ocean Islands; Christopher Helm: London, UK, 2015. [Google Scholar]
- Reddy, S.; Driskell, A.; Rabosky, D.L.; Hackett, S.J.; Schulenberg, T.S. Diversification and the Adaptive Radiation of the Vangas of Madagascar. Proc. R. Soc. B Biol. Sci. 2012, 279, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Jønsson, K.A.; Fabre, P.H.; Fritz, S.A.; Etienne, R.S.; Ricklefs, R.E.; Jørgensen, T.B.; Fjeldså, J.; Rahbek, C.; Ericson, G.P.; Woog, F.; et al. Ecological and Evolutionary Determinants for the Adaptive Radiation of the Madagascan Vangas. Proc. Natl. Acad. Sci. USA 2012, 109, 6620–6625. [Google Scholar] [CrossRef] [PubMed]
- Bristol, R.M.; Fabre, P.H.; Irestedt, M.; Jønsson, K.A.; Shah, N.J.; Tatayah, V.; Warren, B.H.; Groombridge, J.J. Molecular Phylogeny of the Indian Ocean Terpsiphone Paradise Flycatchers: Undetected Evolutionary Diversity Revealed amongst Island Populations. Mol. Phylogenet. Evol. 2013, 67, 336–347. [Google Scholar] [CrossRef]
- Dickinson, E.C.; Christidis, L. The Howard and Moore Complete Checklist of the Birds of the World, 3rd ed.; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Pasquet, E.; Pons, J.M.; Fuchs, J.; Cruaud, C.; Bretagnolle, V. Evolutionary History and Biogeography of the Drongos (Dicruridae), a Tropical Old World Clade of Corvoid Passerines. Mol. Phylogenet. Evol. 2007, 45, 158–167. [Google Scholar] [CrossRef]
- Tateno, M.; Nakamura, M. Breeding Ecology of the Ashy Cuckoo-Shrike Coracinacinerea. Ornithol. Sci. 2009, 8, 147–150. [Google Scholar] [CrossRef]
- Fuchs, J.; Cruaud, C.; Couloux, A.; Pasquet, E. Complex Biogeographic History of the Cuckoo-Shrikes and Allies (Passeriformes: Campephagidae) Revealed by Mitochondrial and Nuclear Sequence Data. Mol. Phylogenet. Evol. 2007, 44, 138–153. [Google Scholar] [CrossRef]
- Eguchi, K.; Yamagishi, S.; Randrianasol, V. The Composition and Foraging Behaviour of Mixed-Species Flocks of Forest-Living Birds in Madagascar. IBIS 1993, 135, 91–96. [Google Scholar] [CrossRef]
- Savage, A.F.; Robert, V.; Goodman, S.M.; Raharimanga, V.; Raherilalao, M.J.; Andrianarimisa, A.; Dé, F.; Ariey, R.; Greiner, E.C. Blood Parasites in Birds from Madagascar. J. Wildl. Dis. 2009, 45, 907–920. [Google Scholar] [CrossRef][Green Version]
- Raharimanga, V.; Soula, F.; Mj, R.; Sm, G.; Randrianarivelojosia, M.; Raharimalala, L.; Jb, D.; Ariey, F.; Robert, V. Hémoparasites des Oiseaux Sauvages à Madagascar. Arch. Inst. Pasteur. De Madag. 2002, 68, 90–99. [Google Scholar]
- Greiner, E.C.; Putnam, M.S.; Goodman, S.M. Blood Parasites from Birds in the Re’serve Naturelle Integrale d’Andringitra, Madagascar. In Zoology 85; Fieldiana: Chicago, IL, USA, 1996; pp. 142–143. [Google Scholar]
- Barraclough, R.K.; Robert, V.; Peirce, M.A. New Species of Haematozoa from the Avian Families Campephagidae and Apodidae. Parasite 2008, 15, 105–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivanova, K.; Zehtindjiev, P.; Mariaux, J.; Dimitrov, D.; Georgiev, B.B. Avian Haemosporidians from Rain Forests in Madagascar: Molecular and Morphological Data of the Genera Plasmodium, Haemoproteus and Leucocytozoon. Infect. Genet. Evol. 2018, 58, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.; Mackenstedt, U.; Woog, F.; Dinkel, A. Avian Malaria on Madagascar: Prevalence, Biodiversity and Specialization of Haemosporidian Parasites. Int. J. Parasitol. 2019, 49, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Valkiunas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bensch, S.; Hellgren, O.; PÉrez-Tris, J. MalAvi: A Public Database of Malaria Parasites and Related Haemosporidians in Avian Hosts Based on Mitochondrial Cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Zídková, L.; Cepicka, I.; Szabová, J.; Svobodová, M. Biodiversity of Avian Trypanosomes. Infect. Genet. Evol. 2012, 12, 102–112. [Google Scholar] [CrossRef]
- Valkiunas, G.; Iezhova, T.A.; Carlson, J.S.; Sehgal, R.N.M. Two New Trypanosoma Species from African Birds, with Notes on the Taxonomy of Avian Trypanosomes. J. Parasitol. 2011, 97, 924–930. [Google Scholar] [CrossRef]
- Šlapeta, J.; Morin-Adeline, V.; Thompson, P.; McDonell, D.; Shiels, M.; Gilchrist, K.; Votýpka, J.; Vogelnest, L. Intercontinental Distribution of a New Trypanosome Species from Australian Endemic Regent Honeyeater (Anthochaera Phrygia). Parasitology 2016, 143, 1012–1025. [Google Scholar] [CrossRef]
- Binkienė, R.; Chagas, C.R.F.; Bernotienė, R.; Valkiūnas, G. Molecular and Morphological Characterization of Three New Species of Avian Onchocercidae (Nematoda) with Emphasis on Circulating Microfilariae. Parasit Vectors 2021, 14, 1–19. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Jones, H.I.; Smith, T.B. Molecular Evidence for Host Specificity of Parasitic Nematode Microfilariae in Some African Rainforest Birds. Mol. Ecol. 2005, 14, 3977–3988. [Google Scholar] [CrossRef]
- Yohannes, E.; Woog, F. A Multi-Isotope and Morphometric Analysis to Uncover Ecological Niche Divergence in Two Endemic Island Birds from Madagascar: The Dark and Common Newtonia (Vangidae). J. Ornithol. 2020, 161, 137–147. [Google Scholar] [CrossRef]
- Wink, M. Use of DNA Markers to Study Bird Migration. J. Ornithol. 2006, 147, 234–244. [Google Scholar] [CrossRef]
- Ciloglu, A.; Ellis, V.A.; Bernotienė, R.; Valkiūnas, G.; Bensch, S. A New One-Step Multiplex PCR Assay for Simultaneous Detection and Identification of Avian Haemosporidian Parasites. Parasitol. Res. 2019, 118, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; Waldenstrom, J.; Bensch, S. A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Ostman, O.; Hansson, B.; Westerdahl, H.; Pinheiro, R.T. Host Specificity in Avian Blood Parasites: A Study of Plasmodium and Haemoproteus Mitochondrial DNA Amplified from Birds. Proc. R. Soc. B Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.A.; Hudspeth, D.S.S. Ribosomal DNA and Phylogeny of the Ascaridoidea (Nemata: Secernentea): Implications for Morphological Evolution and Classification. Mol. Phylogenet. Evol. 1998, 10, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Anderson, T.J.C.; Bandi, C.; Bazzocchi, C.; Genchi, C. A Phylogenetic Analysis of Filarial Nematodes: Comparison with the Phylogeny of Wolbachia Endosymbionts. Parasitology 2001, 122, 93–103. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Mafie, E.; Saito-Ito, A.; Kasai, M.; Hatta, M.; Rivera, P.T.; Ma, X.H.; Chen, E.R.; Sato, H.; Takada, N. Integrative Taxonomic Approach of Trypanosomes in the Blood of Rodents and Soricids in Asian Countries, with the Description of Three New Species. Parasitol Res. 2019, 118, 97–109. [Google Scholar] [CrossRef]
- Musa, S.; Mackenstedt, U.; Woog, F.; Dinkel, A. Untangling the Actual Infection Status: Detection of Avian Haemosporidian Parasites of Three Malagasy Bird Species Using Microscopy, Multiplex PCR, and Nested PCR Methods. Parasitol. Res. 2022, 121, 2817–2829. [Google Scholar] [CrossRef]
- Savage, A.F.; Greiner, E.C. Haemoproteids of the Avian Family Dicruridae (the Drongos). J. Parasitol. 2005, 91, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, J.; Bataillard, A.; Warren, B.H.; Lootvoet, A.; Mirleau, P.; Duval, T.; MilÚ, B.; ThÉbaud, C.; Heeb, P. The Role of Immigration and In-Situ Radiation in Explaining Blood Parasite Assemblages in an Island Bird Clade. Mol. Ecol. 2012, 21, 1438–1452. [Google Scholar] [CrossRef] [PubMed]
- Galen, S.C.; Nunes, R.; Sweet, P.R.; Perkins, S.L. Integrating Coalescent Species Delimitation with Analysis of Host Specificity Reveals Extensive Cryptic Diversity despite Minimal Mitochondrial Divergence in the Malaria Parasite Genus Leucocytozoon. BMC Evol. Biol. 2018, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.F.; Ariey, F.; Greiner, E.C. Hematozoa of the Avian Family Vangidae (the Vangas). J. Parasitol. 2004, 90, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.F.; Ariey, F.; Greiner, E.C. A New Species of Plasmodium from Malagasy Vangas. J. Parasitol. 2005, 91, 926–930. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Jones, H.I.; Smith, T.B. Host Specificity and Incidence of Trypanosoma in Some African Rainforest Birds: A Molecular Approach. Mol. Ecol. 2001, 10, 2319–2327. [Google Scholar] [CrossRef]
- Bennett, G.F.; Earle, R.A.; Squires-Parsons, D. Trypanosomes of Some Sub-Saharan Birds. Onderstepoort J. Vet. Res. 1994, 61, 263–271. [Google Scholar]
- Santolíková, A.; Brzoňová, J.; Čepička, I.; Svobodová, M. Avian Louse Flies and Their Trypanosomes: New Vectors, New Lineages and Host–Parasite Associations. Microorganisms 2022, 10, 584. [Google Scholar] [CrossRef]
- Svobodová, M.; Dolnik, O.V.; Čepička, I.; Rádrová, J. Biting Midges (Ceratopogonidae) as Vectors of Avian Trypanosomes. Parasit Vectors 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Maia da Silva, F.; Marcili, A.; Ortiz, P.A.; Epiphanio, S.; Campaner, M.; Catão-Dias, J.L.; Shaw, J.J.; Camargo, E.P.; Teixeira, M.M.G. Phylogenetic, Morphological and Behavioural Analyses Support Host Switching of Trypanosoma (Herpetosoma) Lewisi from Domestic Rats to Primates. Infect. Genet. Evol. 2010, 10, 522–529. [Google Scholar] [CrossRef]
- Maraghi, S.; Wallbanks, K.R.; Molyneux, D.H. Oral Transmission of Trypanosomes of the Subgenus Herpetosoma from Small Mammals. Parasitol Res. 1995, 81, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Apanius, V. Avian Trypanosomes as Models of Hemoflagellate Evolution. Parasitol. Today 1991, 7, 87–90. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Thomas, N.J.; Hunter, D.B. (Eds.) Parasitic Diseases of Wild Birds; Wiley-Blackwell: New Delhi, India, 2008; ISBN 9780813820811. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).