Abstract
Floral scent is an important trait in plant–pollinator interactions. It not only varies among plant species but also among populations within species. Such variability might be caused by various non–selective factors, or, as has been shown in some instances, might be the result of divergent selective pressures exerted by variable pollinator climates. Cypripedium calceolus is a Eurasian deceptive orchid pollinated mainly by bees, which spans wide altitudinal and latitudinal gradients in mainly quite isolated populations. In the present study, we investigated whether pollinators and floral scents vary among different latitudes. Floral scents of three C. calceolus populations in the Southern Alps were collected by dynamic headspace and analyzed by gas chromatography coupled to mass spectrometry (GC/MS). These data were completed by previously published scent data of the Northern Alps and Scandinavia. The scent characteristics were compared with information on pollinators recorded for present study or available in the literature. More than 80 scent compounds were overall recorded from plants of the three regions, mainly aliphatics, terpenoids, and aromatics. Seven compounds were found in all samples, and most samples were dominated by linalool and octyl acetate. Although scents differed among regions and populations, the main compounds were similar among regions. Andrena and Lasioglossum species were the main pollinators in all three regions, with Andrena being relatively more abundant than Lasioglossum in Scandinavia. We discuss natural selection mediated by pollinators and negative frequency–dependent selection as possible reasons for the identified variation of floral scent within and among populations and regions.
1. Introduction
Animals can drive divergent evolution in plants by applying different selective pressures on plant traits when pollinating them [1,2,3,4]. Among the traits involved in pollinator attraction, flower scent has been shown to mediate or support distance and close–range attraction [5]. The floral scent is known to vary within species, among and within populations [6,7,8], and within individuals across temporal and spatial scales [9]. Although phenotypic variation in scent traits may be caused by variations in environmental factors [10], selection exerted by natural enemies or genetic drift, many studies assume that most of the variation in scent traits is caused by pollinator–mediated natural selection [9].
At least three different scenarios in which pollinator–mediated selection leads to variation in scent (and other) traits are discussed in literature [9]. First, the composition, abundance, and efficiency of pollinators, which Grant and Grant [1] designated as pollinator climate, can vary among populations or with time. Different pollinator climates may lead to interspecific reproductive isolation, potentially followed by divergent speciation and variations in scents among populations/species. This especially is true if locally available pollinators differ in their olfactory preferences. Second, in deceit pollination, the interactions with pollinators may lead to negative frequency–dependent selection, i.e., rare morphs have higher relative fitness than more abundant morphs, leading to intra–population variation in floral traits [11]; but see [12,13,14]. And third, in species with male and female flowers, the selective pressures mediated by pollinators might be different among the sexes, thus leading to intersexual morph variation [9].
Plants spanning large altitudinal or latitudinal gradients likely encounter various different pollinator climates within their distribution area (e.g., [15]), to which they might locally adapt. To test whether plants are adapted to locally varying pollinators, pollinator and scent data are at first compared, and in case of a positive correlation, more in–depth studies follow, often including reciprocal transplant experiments (e.g., [16]). A widespread deceptive and pollinator–dependent orchid that spans wide altitudinal and latitudinal gradients is Cypripedium calceolus L. The species occurs from near sea level to 2500 m a.s.l., and from Northern Italy and the Pyrenees to Scandinavia [17]. In a previous study [12], we investigated the floral scent and the pollinators of C. calceolus along an altitudinal gradient in the Northern Limestone Alps. Scents and pollinator species varied among populations, but not in a concerted manner, as those populations that were most differentiated in altitude differed only in pollinators, but not in scent.
In this study, we explore a latitudinal pattern of C. calceolus’ floral scent and pollinators. We collected new data in the Southern Alps in Italy and expand the geographical scope with literature data from the Northern Alps and from Scandinavia.
2. Materials and Methods
2.1. Plant Species
Cypripedium calceolus L. (C. calceolus ssp. calceolus in [18]) is a flagship species with one of the biggest and most conspicuous flowers of European orchids. The plant is pollen limited [19] and perennial, and distributed across the boreal and temperate zones of Europe and Asia. C. calceolus is found in a variety of habitats including open to medium shaded deciduous and coniferous forests, alpine meadows and rubble, predominantly on calcareous soil [17]. One or two flowers per stem consist of three purple–brown sepals, two similarly colored petals, and a petal called labellum, which is yellow and shoe–shaped to form a trap. The pollen consists of single pollen grains aggregated in a sticky smear. Seeds are with a size of 1.2 × 0.3 mm among the biggest of temperate orchids and are produced in high numbers (6000–16,000 [20]). The plant propagates also vegetatively with short horizontal rhizomes, building patches [17]. The successful pollination of C. calceolus depends on small insects, temporarily trapped in the labellum, and leaving the slippery cavern through a posterior exit opening, thereby passing stigma and anthers, depositing pollen imported from other flowers and gathering new pollen on their back ([21] and references therein). Visitors that are too large can leave the labellum through the entrance opening and are not suitable as pollinators [21].
2.2. Study Sites
The Southern Alps were sampled (scents, pollinators) at three sites in the province of Bozen (Bolzano), Italy, in spring 2017. The populations of Kaltern (Caldaro; 840 m a.s.l.) and Tramin (Termeno; 900 m a.s.l.) were at the dolomitic slopes of the mountain Mendelkamm (Costiera della Mendola) in steep, light pine–beech forests. The population at Bletterbach (1620–1680 m a.s.l.) was situated in a geologically diverse area (volcanics and dolomites) above the Bletterbach George in open coniferous woodland. For the Northern Alps, data from Braunschmid et al. [12] were used and complemented by pollinator and scent data from Annaberg (1350 m a.s.l.) and Taugl (450 m a.s.l.), both in the province of Salzburg, Austria. All the Northern Alps populations studied are situated in the Limestone Alps, and were at altitudes from 450 to 1450 m a.s.l. Scent data for Scandinavia were taken from Bergström et al. [18], who used plants from the Botanical Garden in Uppsala. The pollinator data for Scandinavia were taken from Nilsson [21], Antonelli et al. [22] and Erneberg and Holm [23], who did their investigations in several populations in Sweden and Denmark (Figure 1).
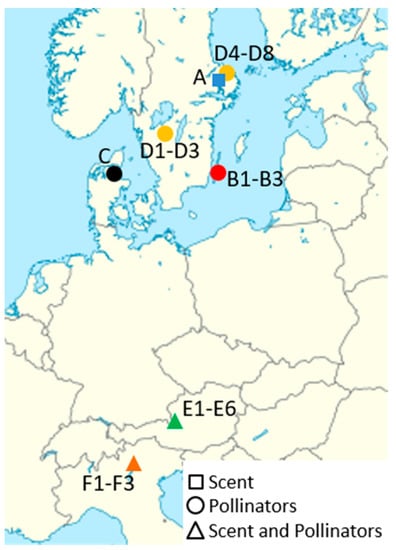
Figure 1.
Regions and study sites at different latitudes in Europe, where data on pollinators (circles), scents (squares), or both (triangles) were collected in the present study or available from literature. Data included in the present study were from Scandinavia ((A) Bergström et al. [18]; (B) Nilsson [21]; (C) Erneberg and Holm [23]; (D) Antonelli et al. [22]), the Northern Alps ((E) Braunschmid et al. [12] and this study), and the Southern Alps ((F) this study). With the exception of A and C, where a single population was sampled, three to six populations were sampled per study area.
2.3. Scent Collection and Analysis
Head space samples of floral volatiles in the Southern Alps were collected in situ during daytime from individual flowers using dynamic headspace methods [6]. Fourteen samples in the Southern Alps and four samples in the Northern Alps (additional to the 60 samples from [12]) from different individuals were collected with the same procedure described in [12]. The adsorbent tubes with the trapped volatiles were analyzed by GC/MS using an automatic thermal desorption system (TD-20, Shimadzu, Japan) coupled to a Shimadzu GC/MS-QP2010 Ultra equipped with a ZB-5 fused silica column (5% phenyl polysiloxane; 60 m, i.d. 0.25 mm, film thickness 0.25 µM, Phenomenex, Torrance, CA, USA), the same as described by Heiduk et al. [24]. The samples were run with a split ratio of 1:1 and a consistent helium carrier gas flow of 1.5 mL/min. The GC oven temperature started at 40 °C, then increased by 6 °C/min to 250 °C and was held for 1 min. The MS interface worked at 250 °C. Mass spectra were taken at 70 eV (EI mode) from m/z 30 to 350. GC/MS data were processed using the GCMSSolution package, Version 4.11 (Shimadzu Corporation 1999–2013).
Identification of the compounds was carried out using the ADAMS, ESSENTIALOILS-23P, FFNSC 2, and W9N11 databases, as well as a database generated from synthetic standards available in the Plant Ecology lab of the University of Salzburg. Based on the compounds detected in C. calceolus, a library was generated and used for semi–automated quantification of samples. Compounds were only included in the study if peak areas in flower samples were at least five times larger than from the green leaf and ambient air controls.
Total scent emission was estimated by injecting known amounts of monoterpenes, aromatics, and aliphatics (added to small adsorbent tubes). The mean response of these compounds (mean peak area) was used to determine the total amount of scent [6]).
For the Scandinavian samples, Bergström et al. [18] trapped scent on Tenax GC and Porapak Q, also followed by GC/MS. They collected three samples between 1980–1983, each from 4–6 cut flowering stalks, and finally reported the mean scent composition.
2.4. Flower Visitor and Pollinator Observation, Collection and Identification
In the flowering season of 2017, on warm and sunny days, two persons went from plant to plant and inspected the flowers for trapped insects at the study sites in the Southern Alps. If insects were found inside the labellum, a perforated transparent plastic bag was put over the flower and the exit mode of the insect—either through the exit opening at the posterior of the flower or back out through the labellum mouth—was observed. After exiting (which sometimes took hours), the insects were captured in the bag and collected for species identification. Immediately after capturing, insects were examined for pollen smear on their back. The Northern and Southern Alps were sampled in the same way, as described in detail in [12]. Observation time accumulated to more than 200 h in the Northern (as given in [12]) and about 40 h in the Southern Alps.
Collection of insects and categorization of pollinators in Scandinavia was comparable to our approach and is described in Nilsson [21], Erneberg and Holm [23], and Antonelli et al. [22]. Observation times were 69 h [21] plus 90 h [23], and is not extractable from Antonelli et al. [22].
In the present paper, insects are classified as pollinators, if they fulfilled the following criteria: they entered the labellum and belong to the same species from which at least one specimen was found in the same population that left the flower through the posterior exit and had pollen smear on its back. The observation times in the regions varied strongly, and therefore the numbers of specimens and species recorded were not analyzed by statistical approaches.
2.5. Statistical Analysis
For analyses of semi–quantitative (i.e., relative amounts of scent components within a flower) and qualitative differences in flower scent among regions and populations, the Bray–Curtis semi–quantitative similarity index and the qualitative Sørensen index were calculated, respectively, to determine pairwise similarities among the individual samples. Based on the obtained similarity matrices, PERMANOVA analyses [25,26] (10,000 permutations) with two factors, populations nested in regions, and region, were performed to test for differences in scent among regions, and within regions, among populations. A PERMDISP analysis [25] tested for differences in dispersion among regions (10,000 permutations). Both analyses were performed with Primer 6.1.16 [26]. Since there was only one data point from Scandinavia, it could not be included in these analyses. Non–metric multidimensional scaling (NMDS), based on the Bray–Curtis similarities, was used to display the semi–quantitative differences in flower scents among regions and populations graphically. The stress value indicates how well the two–dimensional plot represents relationships among samples in multidimensional space. A SIMPER analysis was used to determine the compounds most responsible for variations among populations.
3. Results
3.1. Composition of Flower Scent
The flower scent of C. calceolus along a latitudinal gradient from south of the Alps to Scandinavia contained in a total of 85 compounds, most of which were (tentatively) identified (Table 1). Thirty–two terpenoid substances and 22 aliphatic and 18 aromatic compounds were most numerous, and these three compound classes were also the most abundant ones. C5-branched–chain compounds, nitrogen–containing, and unknown substances were less numerous and contributed together less than a half percent to the total amount of scent emitted. Linalool and octyl acetate were overall by far the most abundant compounds, although both substances varied strongly in relative amount among samples (linalool: 1–73%; octyl acetate: 1–62%).

Table 1.
Occurrence, relative and total absolute amount of scent, and coefficient of variation (CV) of compounds found in the scent of Cypripedium calceolus. Data are based on 78 flower scent samples in the Alps (Southern Alps: 14 samples; Northern Alps: 64 samples, 60 thereof from Braunschmid et al. [12]), complimented by mean values of 3 samples of Uppsala, Scandinavia (taken from Bergström et al. [18]).
3.2. Variations in Scent among Regions and Populations
Quantitative comparisons of samples north and south of the Alps. The mean values of the total amount of scent trapped per flower and minute in the Southern and Northern Alps were quite similar (132 ± 106 and 111 ± 69 ng min−1 ± sd, respectively) and there were no differences in the total amount of scent among these two regions (PERMANOVA PseudoF69.1 = 0.39, p = 0.58). However, differences were obvious among populations (PERMANOVA PseudoF69.7 = 4.9, p < 0.001), and this is true for the Northern Alps (PERMANOVA PseudoF58.5 = 4.7, p = 0.001) as well as the Southern Alps (PERMANOVA PseudoF11.2 = 8.5, p = 0.003).
Qualitative comparisons of samples north and south of the Alps, and of the sample from Uppsala, Scandinavia. Seven compounds were found in all samples (Table 1, Figure 2: benzaldehyde, linalool, heptyl acetate, hexyl acetate, octyl acetate, (Z)-3-hexenyl acetate, and 1-octanol. The compounds (E)-linalool oxide furanoid, 6-methyl-5-hepten-2-one, and (Z)-3-nonenyl acetate were frequently found in the Northern and Southern Alps (>92% of samples) but missing in Uppsala. Other compounds were less frequent, and some were only found in a few samples (Figure 2). In the Northern Alps, 72 compounds were detected, in the Southern Alps 60, and Bergström et al. [18] reported 39 for their study in Uppsala (from whom we excluded seven compounds because these were also present in our leaf samples, in similar amounts as in flower samples). Twenty–two compounds were found in all regions (Table 1, Figure 2). When focusing on the Alps, we found that the spectrum of compounds differed between samples south vs. north of the Alps (PERMANOVA PseudoF69.1 = 2.7, p = 0.03), and these differences cannot be explained by differences in dispersion between the regions (PERMDISP: F1.6 = 0.1, p = 0.77). Overall, there were qualitative differences in scent among alpine populations (PERMANOVA PseudoF69.7 = 4.2, p < 0.001), but only in the Northern Alps (PERMANOVA PseudoF58.5 = 5.3, p = 0.001), and not in the Southern Alps (PERMANOVA PseudoF11.2 = 1.6, p = 0.1).
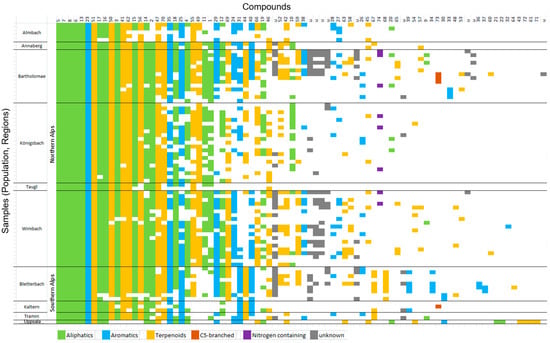
Figure 2.
Visualization of presence/absence of all scent compounds in all samples. The scent compounds are numbered on the x–axes (first line) according to numbers in Table 1. Lines on the y–axes represent the samples, with denotation of their populations and regions of origin. The colors represent compound classes. Data from the Northern Alps (except populations Annaberg and Taugl) were from Braunschmid et al. [12], those from Uppsala (Scandinavia) from Bergström et al. [18].
Semi–quantitative comparisons of samples north and south of the Alps, and of the sample from Uppsala, Scandinavia. Focusing on the Alps, we found that the relative amount of scent (Figure 3; PERMANOVA PseudoF69.1 = 7.6, p = 0.02) and the scent dispersion (Figure 3; PERMDISP: F1.76 = 14.2, p < 0.001) differed between samples south vs. north of the Alps. A Simper analysis showed that the differences in relative scent patterns were mainly due to the most abundant compounds linalool and octyl acetate, which together explained 59% of differences between samples south vs. north of the Alps. In the non–metric multidimensional scaling (NMDS), the samples south of the Alps are grouped at the edge of the samples north of the Alps (Figure 3), mainly due to their relatively higher amount of octyl acetate, whereas the northern samples tended to contain more linalool. The sample from Uppsala differed from the other samples due to its high relative amount of decyl acetate. In addition to the differences in relative scent composition between north and south of the Alps, there were differences in relative scent properties among alpine populations (PERMANOVA PseudoF69.7 = 1.9, p = 0.01), but only in the Northern Alps (PERMANOVA PseudoF58.5 = 2.3, p = 0.01), and not in the Southern Alps (PERMANOVA PseudoF11.2 = 1.2, p = 0.24).
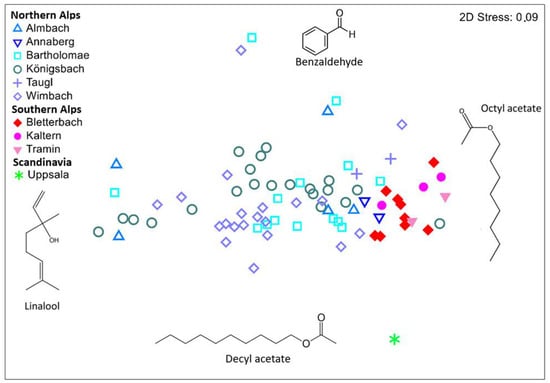
Figure 3.
Non-metric multidimensional scaling (NMDS) were used to visualize semi-quantitative similarities among the single scent samples collected from overall ten different populations in the Southern and Northern Alps, and in Uppsala, Scandinavia. All dots represent single scent samples, with the exception of the Uppsala-data point, which represents the mean of three samples (see Methods). This ordination is based on pairwise Bray–Curtis similarities. Compounds most responsible for ordination of samples as indicated by a SIMPER analysis are also plotted. Data from the Northern Alps (except populations Annaberg and Taugl) were from Braunschmid et al. [12], those from Uppsala, Scandinavia from Bergström et al. [18], and all others were collected for the present study.
3.3. Pollinators
Seventy–five pollinator individuals were caught in the Northern Alps by Braunschmid et al. [12] and 8 in the Southern Alps (Table 2). For Scandinavia, 131 pollinators were reported by Nilsson [21], Erneberg and Holm [23], and Antonelli et al. [22]. All pollinators were bees, except the three syrphid fly species (Platycheirus albimanus, Pipiza austriaca, and Eristalis rupium) and the one sawfly species (Hoplocampa plagiata) reported in [12] for the Northern Alps. Among pollinators, Andrena bees were most numerous in Scandinavia (93 out of 131), Lasioglossum in the Northern Alps (48 out of 75), and both genera occurred with the same numbers in the Southern Alps (4 each out of 8). Three species were recorded as pollinators in all three regions and were abundant in high numbers: Andrena jacobi (= A. carantonica), Lasioglossum calceatum/albipes, and Lasioglossum fulvicorne (see Table 2). Overall most numerous, however, was Andrena haemorrhoa. It occurred with 61 specimens in Scandinavia and was only found three times as flower visitors in the other regions. Some species, such as Andrena haemorrhoa, which were categorized as pollinators in one region were found within the labellum in other regions (contributing not more than 2% to the visitors in these regions), where they only were classified as flower visitors (Table 2).

Table 2.
Pollinators of Cypripedium calceolus were observed at the three regions. with the numbers of individuals per species observed. Data from the Northern Alps were from Braunschmid et al. [12], those from Scandinavia from Nilsson [21], Erneberg and Holm [23], and Antonelli et al. [22].
4. Discussion
Our analyses of the floral scent of Cypripedium calceolus L. from several populations of different regions revealed that plants release, independent of region and population, large numbers of aliphatics, terpenoids, and aromatic compounds, with some compounds present in all samples. However, our analyses also detected qualitative and semi–quantitative, but not quantitative differences in scent characteristics among regions. Within regions, populations in the Southern Alps differed in their quantitative amounts of scent, but not in semi–quantitative and qualitative data. Populations in the Northern Alps differed in all three aspects [12]. The single data set from Scandinavia (Sweden) differed from scent samples of the Alps, however, the small sample size precludes drawing conclusions about the scent at a Scandinavian level. Similar to the scent patterns, variations among regions are also evident in pollinator climate, especially due to differences in the relative importance of Andrena and Lasioglossum bees.
The variability of scent within populations and overall at the species level seems to be high (see Figure 2). However, a quantitative comparison with other species is difficult, as comparable measurements of variability are scarcely published (the few examples include [27,28,29,30]), and if, mostly only stated for the main components, which seems not to be reasonable. This is because it is well known that pollinators not only respond to main but also minor components (e.g., [31]). Here, we present the CV (coefficient of variation) for all fragrance components (see Table 1) for future meta–analyses as proposed by Delle–Vedove et al. [9]. One of the few studies that also gives the CV for all components is [30], which analyzed scents of a deceptive Orchis species. A comparison of both studies shows that the variability in the studied species is similarly high, with mean CVs of 2.2 (C. calceolus) and 2.6 (O. mascula).
We detected some obvious differences in pollinator climate among regions, especially between Scandinavia and the Alps, with the by far most abundant pollinator species in Scandinavia, Andrena haemorrhoa, being not detected as a pollinator, but only rarely as flower visitor, in the Alps. However, given its size, which is in between A. jacobi and L. calceatum/albipes, both of which are pollinators in the Alps, it is likely that it not only visits plants in the Alps, but also acts as pollinator in that region. Despite this main difference and the large geographic region with data from Southern, Central, and Northern Europe, it is interesting that several pollinator species (A. jacobi, L. calceatum/albipes, L. fulvicorne) occurred in all regions. Overall, the number of pollinating species and genera is high in C. calceolus, which makes this deceptive orchid a generalist plant, although the restriction to a certain body size and the necessary agility during the pollination process allows only a subset of the flower visitors to actually pollinate the flowers. Syrphids in particular often visit the flowers but are rarely able to leave the flower through the small posterior exit and thereby receive pollen [12].
Do these findings on pollinator climates explain observed variations in scent patterns of C. calceolus? In the following, we discuss two ways in which natural selection by pollinators can affect variations in scent patterns among populations/regions and within populations, respectively: through different pollinator climates and through frequency–dependent selection [9]. Though caution is needed due to the small sample size in Scandinavia when comparing scents between this region and the Alps, there is a link between scent chemistry and pollinators, which might explain why decyl acetate is more abundant in the Scandinavian “sample” than in any alpine sample. This is because decyl acetate is a quite abundant compound in cephalic secretions of A. haemorrhoa [32], the most abundant pollinator in Scandinavia. In these secretions, which have pheromonal properties, it is much more abundant than octyl acetate [32]. It would be interesting to know whether the high amount of decyl acetate in C. calceolus of Scandinavia was selected by olfactory preferences of A. haemorrhoa. Andrena jacobi, the most numerous Andrena pollinator in the Alps, does not have this compound in its cephalic secretions [33]. This compound is also not known as pheromone of Lasioglossum bees [34], the most abundant pollinators in the Alps. Interestingly, however, this compound elicited antennal responses in flies, Andrena and Lasioglossum bees tested on scents of C. calceolus [12] showing that not only A. haemorrhoa, but also other pollinators perceive and potentially respond (behavior) to this compound. We do not see a link between the chemical differences between the Northern and Southern Alps and the pollinator climates in these regions. Negative frequency–dependent selection may cause intraspecific variability in floral traits and/or mask differences among populations. In the present study, we found significant effects in scent among alpine populations and regions; however, our previous study [12], as well as the present study (Figure 2), also evidenced obvious variations within populations. Although negative frequency–dependent selection is effective within populations, it may also be responsible for differences in scent among populations, as a selection may take different routes in different populations. A recent study [35] investigated frequency–dependent behavior of pollinators in C. calceolus. The authors quantified uni- and multivariate rarity of floral scent characteristics of single flowers and found that the rarity of floral scent phenotypes is not a predictor of whether flowers set fruits. This is an indication that negative frequency–dependent selection is not of relevance in C. calceolus and not responsible for observed variation in floral scent patterns.
Future studies on C. calceolus should consider further factors that might influence scent emissions, such as environmental factors other than pollinators (e.g., soil properties [36,37], temperature [38]), selection by natural enemies [39], and genetic drift [9] to better understand the mechanisms that generate and sustain variation in scent traits in this plant species.
Author Contributions
Conceptualization, S.D. and H.B.; investigation, H.B. and R.G.; writing—original draft preparation, H.B.; writing—review and editing, S.D., R.G. and H.B.; supervision, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the FWF (Austrian Science Fund) grant P32142-B.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Hans Madl (Kaltern), who introduced us to the populations in South Tirol and Ansuela Braunschmid, who helped gathering pollinators. Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grant, V.; Grant, K.A. Flower Pollination in the Phlox Family; Columbia University Press: New York, NY, USA, 1965. [Google Scholar]
- Stebbins, G.L. Adaptive radiation of reproductive characteristics in angiosperms, I: Pollination mechanisms. Annu. Rev. Ecol. Syst. 1970, 1, 307–326. [Google Scholar] [CrossRef]
- Johnson, S.D. Pollinator-driven speciation in plants. In Ecology and Evolution of Flowers; Harder, L.D., Barrett, S.C.H., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 295–310. [Google Scholar]
- Zu, P.; Blanckenhorn, W.U.; Schiestl, F.P. Heritability of floral volatiles and pleiotropic responses to artificial selection in Brassica rapa. New Phytol. 2016, 209, 1208–1219. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef]
- Mant, J.; Peakall, R.; Schiestl, F.P. Does selection on floral odor promote differentiation among populations and species of the sexually deceptive orchid genus Ophrys? Evolution 2005, 59, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.; Sun, M.; Schiestl, F.P. Why do floral perfumes become different? Region-specific selection on floral scent in a terrestrial orchid. PLoS ONE 2016, 11, e0147975. [Google Scholar] [CrossRef] [PubMed]
- Delle-Vedove, R.; Schatz, B.; Dufay, M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann. Bot. 2017, 120, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Majetic, C.J.; Raguso, R.A.; Ashman, T.-L. Sources of floral scent variation. Plant Signal. Behav. 2009, 4, 129–131. [Google Scholar] [CrossRef]
- Gigord, L.D.B.; Macnair, M.R.; Smithson, A. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Proc. Natl. Acad. Sci. USA 2001, 98, 6253–6255. [Google Scholar] [CrossRef]
- Braunschmid, H.; Mükisch, B.; Rupp, T.; Schäffler, I.; Zito, P.; Birtele, D.; Dötterl, S. Interpopulation variation in pollinators and floral scent of the lady’s-slipper orchid Cypripedium calceolus L. Arthropod. Plant. Interact. 2017, 11, 363–379. [Google Scholar] [CrossRef]
- Jersáková, J.; Kindlmann, P.; Renner, S.S. Is the colour dimorphism in Dactylorhiza sambucina maintained by differential seed viability instead of frequency-dependent selection? Folia Geobot. 2006, 41, 61–76. [Google Scholar] [CrossRef]
- Pellegrino, G.; Caimi, D.; Noce, M.E.; Musacchio, A. Effects of local density and flower colour polymorphism on pollination and reproduction in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Plant Syst. Evol. 2005, 251, 119–129. [Google Scholar] [CrossRef]
- Espíndola, A.; Pellissier, L.; Alvarez, N. Variation in the proportion of flower visitors of Arum maculatum along its distributional range in relation with community-based climatic niche analyses. Oikos 2011, 120, 728–734. [Google Scholar] [CrossRef]
- Sun, M.; Gross, K.; Schiestl, F.P. Floral adaptation to local pollinator guilds in a terrestrial orchid. Ann. Bot. 2014, 113, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Cribb, P. The Genus Cypripedium; A Botanical Magazine Monograph; Timber Press: Portland, OR, USA, 1997; ISBN 0-88192-403-2. [Google Scholar]
- Bergström, G.; Birgersson, G.; Groth, I.; Anders Nilsson, L. Floral fragrance disparity between three taxa of lady’s slipper Cypripedium calceolus (Orchidaceae). Phytochemistry 1992, 31, 2315–2319. [Google Scholar] [CrossRef]
- Kull, T. Fruit-set and recruitment in populations of Cypripedium calceolus L. in Estonia. Bot. J. Linn. Soc. 1998, 126, 27–38. [Google Scholar] [CrossRef]
- Kull, T. Cypripedium calceolus L. J. Ecol. 1999, 87, 913–924. [Google Scholar] [CrossRef]
- Nilsson, L.A. Anthecological studies on the Lady’s slipper, Cypripedium calceolus (Orchidaceae). Bot. Not. 1979, 132, 329–347. [Google Scholar]
- Antonelli, A.; Dahlberg, C.J.; Carlgren, K.H.I.; Appelqvist, T. Pollination of the lady’s slipper orchid (Cypripedium calceolus) in Scandinavia-taxonomic and conservational aspects. Nord. J. Bot. 2009, 27, 266–273. [Google Scholar] [CrossRef]
- Erneberg, M.; Holm, B. Bee size and pollen transfer in Cypripedium calceolus (Orchidaceae). Nord. J. Bot. 1999, 19, 363–367. [Google Scholar] [CrossRef]
- Heiduk, A.; Kong, H.; Brake, I.; Von Tschirnhaus, M.; Tolasch, T.; Tröger, A.G.; Wittenberg, E.; Francke, W.; Meve, U.; Dötterl, S. Deceptive Ceropegia dolichophylla fools its kleptoparasitic fly pollinators with exceptional floral scent. Front. Ecol. Evol. 2015, 3. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova + for Primer: Guide to Software and Statisticl Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer v6: User Manual/Tutorial; Primer-E: Plymouth, UK, 2006. [Google Scholar]
- Ackerman, J.D.; Cuevas, A.A.; Hof, D. Are deception-pollinated species more variable than those offering a reward? Plant Syst. Evol. 2011, 293, 91–99. [Google Scholar] [CrossRef]
- Knudsen, J.T. Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the western Amazon. Am. J. Bot. 2002, 89, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, C.C.; Nardella, A.M.; Cozzolino, S.; Schiestl, F.P. Variability in floral scent in rewarding and deceptive orchids: The signature of pollinator-imposed selection? Ann. Bot. 2007, 100, 757–765. [Google Scholar] [CrossRef]
- Salzmann, C.C.; Cozzolino, S.; Schiestl, F.P. Floral scent in food-deceptive orchids: Species specificity and sources of variability. Plant Biol. 2007, 9, 720–729. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee–flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Francke, W.; Reith, W.; Bergström, G.; Tengö, J. Pheromone Bouquet of the Mandibular Glands in Andrena haemorrhoa F. (Hym., Apoidea). Zeitschrift fur Naturforsch. 1981, 36C, 928–932. [Google Scholar] [CrossRef]
- Tengö, J.; Bergström, G. Comparative analyses of complex secretions from heads of Andrena bees (Hym., Apoidea). Comp. Biochem. Physiol. 1977, 57B, 197–202. [Google Scholar] [CrossRef]
- El-Sayed The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 1 December 2020).
- Braunschmid, H.; Dötterl, S. Does the rarity of a flower’s scent phenotype in a deceptive orchid explain its pollination success? Front. Plant Sci. 2020. [Google Scholar] [CrossRef]
- Majetic, C.J.; Fetters, A.M.; Beck, O.M.; Stachnik, E.F.; Beam, K.M. Petunia floral trait plasticity in response to soil nitrogen content and subsequent impacts on insect visitation. Flora Morphol. Distrib. Funct. Ecol. Plants 2017, 232, 183–193. [Google Scholar] [CrossRef]
- Sletvold, N.; Grindeland, J.M.; Agren, J. Vegetation context influences the strength and targets of pollinator-mediated selection in a deceptive orchid. Ecology 2013, 94, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.A.; Burkle, L.A.; Manson, J.S.; Runyon, J.B.; Trowbridge, A.M.; Zientek, J. Global change effects on plant–insect interactions: The role of phytochemistry. Curr. Opin. Insect Sci. 2017, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Knauer, A.C.; Schiestl, F.P. The effect of pollinators and herbivores on selection for floral signals: A case study in Brassica rapa. Evol. Ecol. 2017, 31, 285–304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).