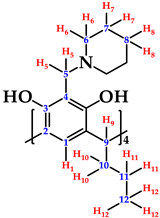
Abstract
Calix[4]resorcinarenes are polyhydroxylated macrocyclic compounds with four units of resorcinol. These compounds can be derivatized through modifications at the upper rim, allowing reactivity with secondary amines to produce Mannich base derivatives via Mannich-type aminomethylation reactions. In this paper, we report the reaction of C-tetra(propyl)calix[4]resorcinarene with piperidine in acetonitrile. The aminomethylated compound C-2,8,14,20-tetra(propyl)-5,11,17,23-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene was obtained with a 52% yield, with an exact mass of 1044.6994 u and a mass error of 7.6 ppm. The reaction progress and product formation were monitored by RP-HPLC, and the compound was characterized using LC ESI-TOF/MS, one- and two-dimensional 1H and 13C NMR, and FTIR spectroscopy. Chromatographic and spectroscopy data are presented and discussed.
1. Introduction
The Mannich reaction is a powerful tool in the field of organic chemistry for the formation and incorporation of C-C bonds in different molecules. This amino alkylation is a three-component nucleophilic condensation reaction between an acidic hydrogen compound, a non-enolizable aldehyde, e.g., formaldehyde, and a secondary amine or amide. The condensation produces a Mannich base, in which a hydrogen atom is replaced by an aminomethyl group [1,2,3]. In calix[4]resorcinarenes, polyhydroxylated macrocyclic molecules synthesized from resorcinol and aldehyde, which contain aromatic active hydrogens in the ortho position on the upper rim, can be replaced by the addition of an aminomethyl derivative in order to obtain the corresponding Mannich base. This process involves, as a first step, the reaction between amine and a methylation agent, e.g., formaldehyde, generating the hemiaminal intermediate, a subsequent dehydration, and the formation of an iminium cation. The second step involves a reaction in which the aromatic phenolic ring, rich in electrons in the calix[4]resorcinarene, attacks the iminium cation via an electrophilic aromatic substitution (SEAr). In this type of substitution, the electron-rich aromatic ring, in the presence of an electrophile, forms a cationic intermediate, which recovers its aromaticity by the loss of a hydrogen atom in position 2. Therefore, the corresponding Mannich base is formed in the ortho position by replacing aromatic hydrogen (Figure 1) [2,4,5]. When primary amines are used, the product reaction is generally a benzoxazine, due to a second aminomethylation step and the intramolecular nucleophilic attack of a phenolic hydroxyl group on the iminium intermediate. In some cases, the Mannich base has been obtained using primary amines, depending on the pH and the solvent used [6].
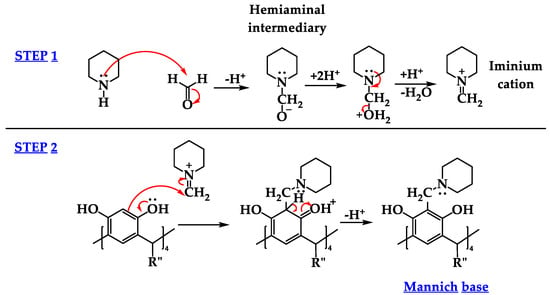
Figure 1.
Reaction mechanism of Mannich base formation by Mannich-type aminomethylation reaction, specifically at the resorcinol rings of the cyclotetramer resorcinarene [2,4,5].
Mannich bases have been obtained through Mannich-type aminomethylation reactions using calix[4]resorcinarenes and secondary amines as precursors in ethanolic solvent mixtures. An example of this process is the reaction of resorcinarenes such as tetra(nonyl)calix[4]resorcinarene or tetra(4-hydroxyphenyl)calix[4]resorcinarene which can be functionalized with L-proline [7,8,9].
Therefore, in this investigation, we present the synthesis of the incorporation of a heterocyclic piperidine amine into C-tetra(propyl)calix[4]resorcinarene by a Mannich aminomethylation reaction, obtaining a highly symmetrical 2-substituted cyclotetramer Mannich base, using acetonitrile (ACN), an aprotic polar solvent. RP-HPLC, LC-MS, and spectroscopy analyses are presented and discussed.
2. Results and Discussion
In the present study, we particularly wanted to see if the use of a polar aprotic solvent as an alternative to protic solvents such as ethanol, or more common mixtures such as EtOH:H2O or EtOH:C6H6, commonly used for aminomethylation reactions [10], has any effect on the substitution, causing, for example, partial substitution at position 2 of the phenolic rings, no Mannich base formation, or an effect on the conformational properties of the product(s), taking as a starting point the symmetric C-tetra(propyl)calix[4]resorcinarene (1), which is stabilized in the crown conformation by the formation of intramolecular hydrogen bonds [6,11].
The synthesis of the precursor C-tetra(propyl)calix[4]resorcinarene (1) was performed following a previously reported procedure (see Section 3.2) [12]. The IR spectrum data of 1 show bands corresponding to functional groups in the resorcinarene precursor, notably the phenolic O–H stretching vibrations. 1H and 13C NMR spectra show only seven proton and seven carbon signals. The limited number of signals observed in both the aromatic (downfield) and aliphatic (upfield) regions indicates that the majority of the carbon nuclei are chemically equivalent. Consequently, compound 1 is probably highly symmetrical, corresponding to C4v point group symmetry, which corresponds to an rccc-crown conformation. In other conformations, a higher number of signals could be observed because of the spatial arrangement of the resorcinol units and the orientation of the substituents, resulting in a higher number of non-equivalent atoms. For example, in the C-tetra(methyl)calix[4]resorinarene rccc-crown (C4v), when compared to the boat (C2v) and chair (C2h) conformers, a higher number of 1H-NMR signals is observed in the corresponding spectrum, suggesting conformational differences in each case [13].
After obtaining precursor 1, the upper rim was functionalized by Mannich-type aminomethylation reaction using piperidine as the precursor amine. Since piperidine is a secondary cyclic amine, the reaction at position two in the aromatic units of calix[4]resorcinarene should correspond to a Mannich base. The reaction, using acetonitrile as a solvent, occurred at room temperature, in a short time and it was not necessary to use catalysts or reflux conditions.
The solid product (2) was analyzed by RP-HPLC at 280 nm (Figure 2a,b). A comparison of the chromatographic profile of calix[4]resorcinarene precursor 1 (Figure 2a) and the precipitated pink solid compound 2 showed a shorter retention time in RP-HPLC, suggesting the higher hydrophilicity of compound 2 than that of precursor 1, due to the presence of nitrogen atoms in the aminomethylated compound. The LC ESI-TOF/MS isotopic pattern of species [M+H]1+ is shown in Figure 2c.
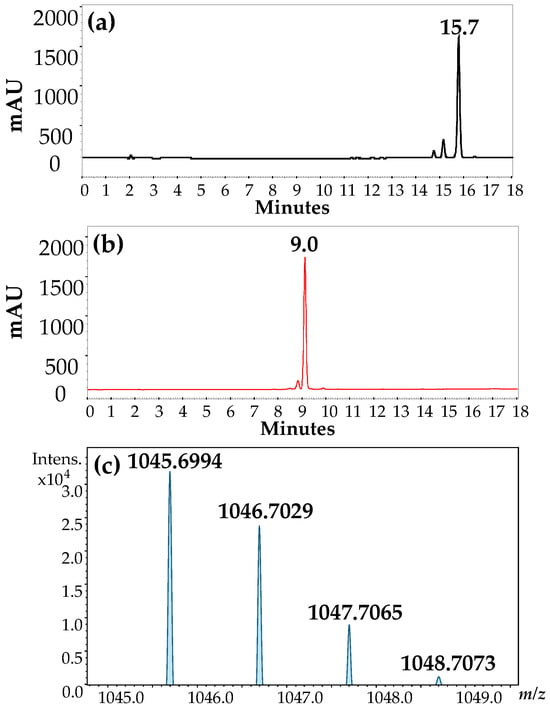
Figure 2.
RP-HPLC chromatogram: (a) C-tetra(propyl)calix[4]resorcinarene precursor (1), (b) C-2,8,14,20-tetra(propyl)-5,11,17,23-tetrakis(N-(piperidin)methyl)calix[4]resorcinarene product (2), and (c) ESI-TOF/isotopic distribution of [M+H]+ of compound 2, with mass error of 7.6 ppm.
The resulting product showed a mass isotopic pattern with a [M+H]+ signal of m/z = 1045.6994. The experimental mass (Expmass) was calculated to be 1044.6994 u, which was compared with the theoretical mass—M: 1044.6915 u—with an error of 7.6 ppm, confirming the successful formation of the tetra-aminomethylated product between 1 and piperidine. Multiple charged ions were observed at m/z = 523.3533, corresponding to [M+2H]2+, and m/z = 349.2382, corresponding to [M+3H]3+. These ions result from the protonation of the nitrogen atoms in the ESI system, which is expected due to the presence of amines in the upper ring, as depicted in the structure in Figure 3.
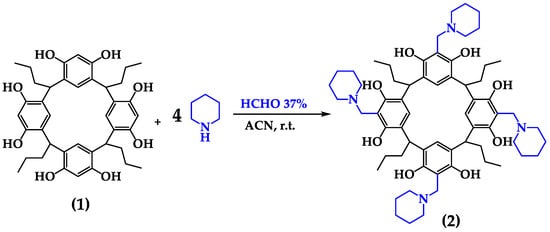
Figure 3.
Reaction of functionalization of C-tetra(propyl)calix[4]resorcinarene (1) with piperidine in ACN solvent. The Mannich base tetra-substitutive product is C-2,8,14,20-tetra(propyl)-5,11,17,23-tetrakis(N-(piperidin)methyl)calix[4]resorcinarene (2).
The IR spectroscopic data are reported in Section 3.3, and the 1D and 2D NMR data are presented in Table 1. The bands observed in FTIR are evidence of some characteristic functional groups of the aminomethylated compound 2. Bands were observed that correspond to the stretches of phenolic hydroxyl groups (-OH, 3200 cm−1), aliphatic carbons and a methine bridge on the lower edge (-CH2, -CH, 2930 and 2856 cm−1), and the bonds of the tri-substituted cyclic amine derived from functionalization with piperidine (C-N, 1111 cm−1), in the ortho position in the phenolic rings. Other intense bands corresponded to the C=C stretches of the aromatic rings (1609 and 1450 cm−1).

Table 1.
Two-dimensional NMR correlation for Mannich base compound 2.
As shown in Table 1, the ¹H NMR spectrum shows nine signals corresponding to the spin–spin couplings of the protons and the disappearance of one of the two signals in the aromatic range, confirming the substitution in the ortho position, and the ¹3C NMR spectrum exhibits twelve signals corresponding to the chemically differentiated carbons. The signal at 151.0 ppm is very weak but is identified through the vicinal coupling between H1 and C3, which is linked to the hydroxyl group in the ¹H-¹3C HMBC spectrum. The two-dimensional ¹H-¹3C HSQC spectrum shows nine carbons with hydrogen, as shown for the proposed structure. Note that the chemical shifts of C5 and C6 (δ = 53.7 and 55.9 ppm) and the respective hydrogen ones are within the ranges of aliphatic C-N reported in the literature [14]. In addition, the HMBC experiment showed long-distance heteronuclear correlations (2J, 3J, and 4J), establishing connectivity between the aromatic proton H1 (7.11 ppm) and the aromatic carbons C2 (124.0 ppm), C3 (151.0 ppm), and C4 (107.0 ppm), and the methine carbon C9 (33.1 ppm). The connectivity between H6 (3.74 ppm) of the piperidine ring, the methylene carbon C5 (53.7 ppm), the ortho-substituted aromatic carbon C4 (107.0 ppm), produced by the Mannich-type aminomethylation reaction, and C3 was confirmed. This corresponds to the molecular structure presented and confirms the obtainment of the Mannich base 2.
The HSQC experiment showed that the geminal protons H5 (2.05 ppm) and H5′ (2.97 ppm) at the C5 methylene bridge are diastereotopic. This difference in their chemical shifts can be explained by the two methylene bridge hydrogens being in different environments, i.e., axial and equatorial types, and consequently, they are not equivalent [15]. The 1H-1H COSY couplings are as expected for the alkyl propyl chain of the starting resorcinarene lower edge. The fact that there is the minimum number of equivalent signals in the 1H and 13C spectra and no duplications for the same carbon or hydrogen indicates that the molecule is symmetric, tetra-substituted, and maintains the rccc-crown conformation of the precursor resorcinarene 1.
The adopted methodology did not use basic catalysts, as was carried out in other reported methodologies [8], and the use of an aprotic polar solvent did not affect the stabilization of the intermediates, so the reaction was carried out with good yields (52%) and worked in an open atmosphere, in contrast to methodologies that require the use of a N2 atmosphere for cyclic amines with alkyl calix[4]resorcinarenes [12]. The synthesis was very clean, since no additional purification processes were required. To the best of our knowledge, this is the first time that this derivative has been obtained and characterized.
3. Materials and Methods
3.1. General Experimental Information
All the reagents and solvents used in the synthesis were of analytical grade and were used without further purification. Resorcinol, butyraldehyde, trifluoroacetic acid (TFA), acetonitrile (ACN), and piperidine were purchased from Sigma-Aldrich (St. Louis, MO, USA). CDCl3 was obtained from Merck (Darmstadt, Germany). FTIR-ATR analysis was performed using a Thermo Nicolet iS5 FTIR equipped with an ATR iD7 accessory, and the absorption was recorded in cm−1. The nuclear magnetic resonance spectrum of 1H NMR was obtained at 400 MHz and that of 13C NMR at 101 MHz on a Bruker Avance 400 instrument, using CDCl3 as a sample solvent. Chemical shifts (δ) are reported in ppm, using the solvent residual signal.
3.2. General Procedure for Synthesis of C-tetra(propyl)calix[4]resorcinarene
Resorcinol (4 mmol) was dissolved in 10 mL of ethanol and 4 mL of 37% hydrochloric acid with stirring. Separately, butanal (4 mmol) was dissolved in 6.5 mL of ethanol and added dropwise to the reaction under stirring. The mixture was refluxed for 6 h, and a clear yellow solution was obtained. The addition of 50 mL of Type I water induced the formation of a yellow precipitate. The yellow precipitate was separated by filtration, washed three times with water, and dried under reduced pressure. The product was characterized using RP-HPLC, 1H NMR, 13C NMR, and ATR-FTIR.
C-2,8,14,20-tetra(propyl)calix[4]resorcinarene (1) was obtained as a pale orange solid with 92% yield. FTIR (ATR/cm−1): 3270 ν(O–H), 2954–2867 ν(C–H), 1200–1100 ν(C–O) 1H NMR, DMSO-d6, (δ, ppm): 0.89 (t, J = 7.3 Hz, 12H, CH3), 1.19 (m, J = 7.4 Hz, 8H, CH2), 2.08 (q, 8H, CH2), 4.22 (t, 4H, CH), 6.14 (s, 4H, CH aromatic), 7.24 (s, 4H, CH aromatic), 8.92 (s, 4H, OH); 13C NMR, DMSO-d6, (δ, ppm): 151.4 (C of -OH), 124.8 (aromatic C), 123.0 (aromatic C), 102.1 (aromatic C), and 35.5 (methine C), 32.4, 20.5, 13.6 (aliphatic propyl chain C).
3.3. General Procedure for the Synthesis of C-2,8,14,20-tetra(propyl)-5,11,17,23-tetrakis(N-(piperidin)methyl)calix[4]resorcinarene
C-tetra(propyl)calix[4]resorcinarene (1 mmol) was dissolved in 5 mL of ACN, and 400 μL (10 mmol) of formaldehyde was added. A solution of 2 mL of piperidine (6 mmol) in ACN was added dropwise. The reaction mixture was stirred constantly at room temperature for 6 h. The precipitate was separated by filtration and dried under vacuum. The final product was analyzed and characterized via RP-HPLC, LC ESI-TOF/MS, 1H NMR (400 MHz, CDCl3, 297 K), 13C NMR (101 MHz, CDCl3, 297 K), ¹H-¹H COSY (CDCl3, 298 K), ¹H-¹3C HMBC (CDCl3, 297 K), ¹H-¹3C HSQC (CDCl3, 297 K), and ATR-FTIR.
C-2,8,14,20-tetra(propyl)-5,11,17,23-tetrakis(N-(piperidin)methyl)calix[4]resorcinarene (2) was obtained as a pale pink solid with a 52% yield. FTIR (ATR/cm−1): 3200 ν(O-H), 2930, 2856 cm−1 ν(C-H aliphatic), 1609, 1450 cm−1 ν(C=C Ar), 1111 ν(C-N). 1H NMR, CDCl3, (δ, ppm): 0.98 (t, J = 7.3 Hz, 12H, CH3), 1.35 (m, J = 7.4 Hz, 2H, CH2), 1.62 (broad s, 8H, CH2 of piperidine), 1.75 (broad s, 8H, CH2 of piperidine), 2.05 (broad s, 4H, geminal hydrogen, CH2), 2.17 (broad s, J = 8.0 Hz, 8H, CH2), 2.97 (broad s, 4H, geminal hydrogen, CH2), 3.74 (broad s, 8H, CH2 of piperidine), 4.34 (t, J = 7.9 Hz, 4H, CH methine hydrogen), 7.11 (s, 4H, aromatic CH); 13C NMR, CDCl3, (δ, ppm): 124.0 (aromatic C), 122.0 (aromatic C), 107.0 (ortho-substituted aromatic carbon relative to the hydroxyls), 55.9 (C-N heterocyclic piperidine), 53.7 (C-N, aminomethylation), 35.5 (CH2, aliphatic), 33.1 (CH, methine), 25.6, 23.9 (C, heterocyclic piperidine), 21.3 (CH2, aliphatic), 14.2 (CH3, aliphatic). The ESI-TOF/MS spectrum showed a signal at m/z = 1045.6994 corresponding to [M+H]+, m/z = 523.3533 corresponding to [M+2H]2+, and m/z = 349.2382 corresponding to [M+3H]3+. Theoretical mass: 1044.6915 u; experimental mass (Expmass): 1044.6994 u; Δm: 7.6 ppm.
3.4. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) Analysis
RP-HPLC analyses were performed on a Hitachi Primade 1410 with a DAD detector set to 280 nm. The mobile phase was ACN-TFA 0.05% (B) in H2O-TFA 0.05% (A). The gradient elution was 20/20/100/100/20/20% B at 0/1/18/21/21.1/24 min, and the flow rate was 1.0 mL/min, with a Phenomenex Luna C8 column (100 × 4.6 mm, 5 μm, 100 Å).
3.5. LC-MS Methodologies
Compound analysis was performed using an Impact II LC-MS (Q-TOF) (Bruker Daltonik; Bremen, Germany) equipped with an electrospray ionization (ESI) source, operating in the positive mode. The chromatographic conditions were as follows: Intensity Solo C18 column (2.1 × 100 mm, 1.8 μm) (Bruker Daltonik; Bremen, Germany), temperature of 40 °C, and flow rate of 0.250 mL/min. The mobile phase consisted of water (A) and acetonitrile (B), each containing 0.1% formic acid. The gradient program was as follows: 5/5/95/95/5/5% B in 0/1/11/13/13.1/15 min. The ESI source conditions were as follows: endplate displacement 500 V, capillary at 4500 V, nebulizer at 1.8 bar, dry gas (nitrogen) 8.0 L/min, dry gas temperature 220 °C. Auto MS/MS scanning mode was used with a mass range of 20–2000 m/z, spectral rate of 6 Hz, and a collision energy of 5.0 eV.
Supplementary Materials
Figure S1: 1H NMR C-tetra(propyl)-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene; Figure S2: 13C NMR C-tetra(propyl)-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene; Figure S3: H-H COSY C-tetra(propyl)-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene Figure S4: ¹H-¹3C HSQC C-tetra(propyl)-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene; Figure S5: ¹H-¹3C HMBC C-tetra(propyl)-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene; Figure S6: ATR-FTIR: C-tetra(propyl)-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene; Table S1: MS data C-tetra(propyl)-tetrakis(N–(piperidine)methyl)calix[4]resorcinarene.
Author Contributions
Conceptualization, V.A.N.-R., M.M. and Z.J.R.-M.; methodology, V.A.N.-R.; formal analysis, V.A.N.-R., M.M. and Z.J.R.-M.; investigation, V.A.N.-R.; data curation, M.M. and V.A.N.-R.; writing original draft preparation, V.A.N.-R., M.M. and Z.J.R.-M.; writing—review and editing, Z.J.R.-M. and M.M.; supervision, M.M. and Z.J.R.-M.; project administration, M.M. and Z.J.R.-M.; funding acquisition, M.M. and Z.J.R.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINCIENCIAS, Project “Diseño y obtención de nuevos agentes antibacterianos basados en dendrímeros péptido-resorcinareno: Una alternativa para combatir la resistencia bacteriana”, under grant number RC-846-2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank MINCIENCIAS and the Universidad Nacional de Colombia-Sede Bogotá for the doctoral scholarship “Asistente Docente”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Subramaniapillai, S.G. Mannich Reaction: A Versatile and Convenient Approach to Bioactive Skeletons. J. Chem. Sci. 2013, 125, 467–482. [Google Scholar] [CrossRef]
- Tramontini, M.; Angiolini, L. Further Advances in the Chemistry of Mannich Bases. Tetrahedron 1990, 46, 1791–1837. [Google Scholar] [CrossRef]
- Yamali, C.; Gul, H.I.; Sakagami, H.; Supuran, C.T. Synthesis and Bioactivities of Halogen Bearing Phenolic Chalcones and Their Corresponding Bis Mannich Bases. J. Enzyme Inhib. Med. Chem. 2016, 31, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Chirachanchai, S.; Phongtamrug, S.; Laobuthee, A.; Tashiro, K. Mono-Substituted Phenol-Based Benzoxazines: Inevitable Dimerization Via Self-Termination and Its Metal Complexation. Handb. Benzoxazine Resins 2011, 111–126. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 7th ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Kuberski, B.; Pecul, M.; Szumna, A. A Chiral “Frozen” Hydrogen Bonding in C4-Symmetric Inherently Chiral Resorcin[4]Arenes: NMR, X-Ray, Circular Dichroism, and Theoretical Study (Eur. J. Org. Chem. 18/2008). Eur. J. Org. Chem. 2008, 2008, 3027. [Google Scholar] [CrossRef]
- Velásquez-Silva, B.A.; Castillo-Aguirre, A.; Rivera-Monroy, Z.J.; Maldonado, M. Aminomethylated Calix[4]Resorcinarenes as Modifying Agents for Glycidyl Methacrylate (GMA) Rigid Copolymers Surface. Polymers 2019, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Aguirre, A.; Maldonado, M. Preparation of Methacrylate-Based Polymers Modified with Chiral Resorcinarenes and Their Evaluation as Sorbents in Norepinephrine Microextraction. Polymers 2019, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Pietraszkiewicz, M.; Prus, P.; Bilewicz, R. PH Dependent Enantiomeric Recognition of Amino Acids by Mannich-Type Calix[4]Resorcinarenes in Langmuir Monolayers. Pol. J. Chem. 1999, 73, 1845–1853. [Google Scholar]
- Luostarinen, M.; Shivanyuk, A.; Rissanen, K. Partial Aminomethylation of Resorcarenes. Org. Lett. 2001, 3, 4141–4144. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Silva, A.; Cortés, B.; Rivera-Monroy, Z.J.; Pérez-Redondo, A.; Maldonado, M. Crystal Structure and Dynamic NMR Studies of Octaacetyl-Tetra(Propyl)Calix[4]Resorcinarene. J. Mol. Struct. 2017, 1137, 380–386. [Google Scholar] [CrossRef]
- Matsushita, Y.; Matsui, T. Synthesis of Aminomethylated Calix[4]Resorcinarenes. Tetrahedron Lett. 1993, 34, 7433–7436. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Maldonado, M.; Rivera-Monroy, Z.J. Efficient Separation of C-Tetramethylcalix[4]Resorcinarene Conformers by Means of Reversed-Phase Solid-Phase Extraction. ACS Omega 2023, 8, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Silverstein, R.; Webster, F.; Kiemle, D.; Brice, D. Spectrometric Identification of Organic Compounds, 8th ed.John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 978-0-470-61637-6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).