Abstract
Functionally substituted 1,2,5-oxadiazole 2-oxides (furoxans) are important pharmaceutical scaffolds used for the preparation of various pharmacologically active substances. Furoxans bearing hydrazone functionality are considered as promising drug candidates for the treatment of neglected diseases. However, pharmacologically oriented hydrazones derived from (furoxanyl)amidrazones and acetylfuroxans have remained unknown so far. In this communication, a simple method for the synthesis of 4-amino-3-(1-{[amino(3-methyl-2-oxido-1,2,5-oxadiazol-4-yl)methylene]hydrazinylidene}ethyl)-1,2,5-oxadiazole 2-oxide is described. The structure of the synthesized compound was established by elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR and IR spectroscopy.
1. Introduction
Hydrazones are versatile bioactive molecules displaying a wide range of pharmacological activities, including antibacterial, antitubercular, antifungal, anticancer, etc. [1,2]. Such compounds usually have acceptable hydrolytic stability, low toxicity and may serve various biomedical applications [3,4]. In addition, hydrazones are useful synthons for the formation of carbon–nitrogen and nitrogen–heteroatom bonds, which is highly relevant for the creation of new methods of constructing functionally substituted nitrogen heterocycles [5].
In a series of nitrogen heterocycles, 1,2,5-oxadiazoles N-oxides (furoxans) became emergent scaffolds with strong application potential in various areas. Furoxans correspond to an important subclass of heterocyclic pharmaceutical ingredients capable of exogenous release of nitric oxide (NO) [6,7]. NO is a crucial gaseous signaling molecule that mediates various physiological processes and may be useful for cancer treatment [8,9,10,11]. Due to described NO-releasing properties, furoxans have a wide range of pharmacological activities including antiparasitic [12,13,14,15], antiaggregant [16,17,18] and anticancer [19,20] types of activity. Recently, a potential application of arylazofuroxans as organic photoswitchable NO-donors was proposed [21]. In addition, furoxan-based hydrazones are considered as promising drug candidates for the treatment of various neglected diseases including tuberculosis, leishmaniosis, schistosomiasis and Chagas’ disease [12,13,14,15,22]. Herein, we report the synthesis of 4-amino-3-(1-{[amino(3-methyl-2-oxido-1,2,5-oxadiazol-4-yl)methylene]hydrazinylidene}ethyl)-1,2,5-oxadiazole 2-oxide 1, which can be considered as a promising pharmacologically active compound for the treatment of neglected diseases.
2. Results and Discussion
Hydrazones are usually prepared via condensation of substituted hydrazines with carbonyl compounds. However, furoxanylhydrazines are unstable compounds that cannot be isolated, only generated in situ under rather harsh conditions [23]. Therefore, we decided to investigate the reactivity of more convenient and bench-stable (furoxanyl)amidrazones for the preparation of the corresponding hydrazones. (3-Methylfuroxan-4-yl)amidrazone 2 [24] and 3-acetyl-4-aminofuroxan 3 [25] were chosen as readily available compounds in the series of functionally substituted furoxan derivatives, which can be synthesized on a large scale. The condensation of substrates 2 and 3 was performed under mild reaction conditions in MeCN at 25 °C and resulted in the formation of target product 1 in a good yield (Scheme 1).
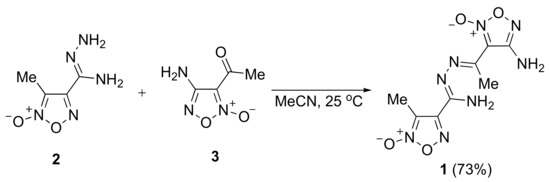
Scheme 1.
Synthesis of hydrazone 1.
The structure of target product 1 was fully confirmed by elemental analysis, high-resolution mass spectrometry and 1H, 13C NMR and IR spectroscopy (see Supplementary Materials). HRMS and elemental analysis confirm the chemical formula of compound 1. The 1H NMR spectrum of 1 showed signals of both methyl groups at 2.36 ppm (methyl group at the furoxan ring) and 2.41 ppm (methyl group of the hydrazone functionality) and of both amino groups at 6.55 ppm (amino group at the furoxan ring) and 7.52 ppm (amino group of the amidrazone functionality). Characteristic signals of C-3 furoxan carbon atoms were observed in the 13C NMR spectrum at 109.8 and 113.3 ppm. The IR spectrum demonstrated characteristic bands of amino groups (3501 and 3386 cm−1), C=N double bonds (1633 cm−1) and furoxan rings (1598 and 1566 cm−1).
In conclusion, we synthesized a new furoxan-based hydrazone representative, namely 4-amino-3-(1-{[amino(3-methyl-2-oxido-1,2,5-oxadiazol-4-yl)methylene]hydrazinylidene}ethyl)-1,2,5-oxadiazole 2-oxide 1, which is a promising pharmacologically oriented substance and a useful precursor of various polynitrogen heterocyclic systems.
3. Materials and Methods
General. All reactions were carried out in well-cleaned, oven-dried glassware with magnetic stirring. 1H and 13C NMR spectra were recorded on a Bruker AM-300 (300.13 and 75.47 MHz, respectively) spectrometer (Billerica, MA, USA) and referenced to residual solvent peaks. The chemical shifts are reported in ppm (δ); coupling constants, J, are reported in Hertz. The IR spectra were recorded on a Bruker “Alpha” spectrometer (Münich, Germany) in the range 400–4000 cm−1 (resolution 2 cm−1). Elemental analyses were performed by the CHN Analyzer Perkin-Elmer 2400 (Waltham, MA, USA). High-resolution mass spectra were recorded on a Bruker microTOF spectrometer (Münich, Germany) with electrospray ionization (ESI). All measurements were performed in a positive (+MS) ion mode (interface capillary voltage: 4500 V) with scan range m/z: 50–3000. External calibration of the mass spectrometer was performed with Electrospray Calibrant Solution (Fluka). A direct syringe injection was used for all analyzed solutions in MeCN (flow rate: 3 μL min−1). Nitrogen was used as a nebulizer gas (0.4 bar) and dry gas (4.0 L min−1); the interface temperature was set at 180 °C. All spectra were processed using the Bruker DataAnalysis 4.0 software package (Billerica, MA, USA). Analytical and preparative thin-layer chromatography (TLC) was carried out on Merck 25 TLC silica gel 60 F254 aluminum sheets. The visualization of the TLC plates was accomplished with a UV light. All solvents were purified and dried using standard methods prior to use. All standard reagents were purchased from Aldrich or Acros Organics and used without further purification. Amidrazone 2 [24] and 3-acetyl-4-aminofuroxan 3 [25] were prepared according to published procedures.
Synthesis of 4-amino-3-(1-{[amino(3-methyl-2-oxido-1,2,5-oxadiazol-4-yl)methylene]hydrazinylidene}ethyl)-1,2,5-oxadiazole 2-oxide 1. 3-Acetyl-4-aminofuroxan 3 (286 mg, 2 mmol) was added to a magnetically stirred solution of amidrazone 2 (314 mg, 2 mmol) in MeCN (5 mL) at 25 °C. The reaction mixture was stirred at 25 °C for 10 h until full consumption of the starting material according to TLC data (eluent CHCl3-EtOAc, 75:25). Then, the solvent was evaporated, and the crude product was purified by preparative TLC (eluent CHCl3-EtOAc, 80:20). Yield 412 mg (73%), yellow needles, mp. 229–230 °C (recrystallized from MeCN). Rf 0.12 (CH2Cl2-EtOAc, 90:10), 0.30 (CHCl3-EtOAc, 80:20), 0.55 (CHCl3-EtOAc, 50:50). IR spectrum (KBr), ν, cm−1: 3501 (NH), 3386 (NH), 2958 (CH), 2931 (CH), 1633 (C=N), 1598 (furoxan), 1566 (furoxan), 1502, 1457, 1349, 1108, 1040, 858. 1H NMR (DMSO-d6, ppm): 2.36 (s, 3H), 2.41 (s, 3H), 6.55 (s, 2H), 7.52 (s, 2H). 13C NMR (DMSO-d6, ppm): 10.4, 14.4, 109.8, 113.3, 148.8, 153.0, 153.1, 156.9. HRMS (ESI) Calcd. for C8H10N8NaO4 [M + Na]+: 305.0717, found: 305.0721. Anal. calcd. for C8H10N8O4 (%): C, 34.05; H, 3.57; N, 39.71. Found (%): C, 33.92; H, 3.65; N, 39.53.
Supplementary Materials
The following are available online: copies of 1H and 13C NMR, IR, HRMS for the compound 3.
Author Contributions
Conceptualization, A.S.K. and L.L.F.; methodology, M.A.E.; investigation, M.A.E. and A.S.K.; data curation, M.A.E. and A.S.K.; writing—original draft preparation, L.L.F.; writing—review and editing, L.L.F.; supervision, L.L.F.; project administration, L.L.F.; funding acquisition, L.L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data obtained in this project are contained within this article and available upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popiolek, L. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Rahmat Ali, M.; Mumtaz Alam, M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, J.; Milkowski, S. The Use of Hydrazones for Biomedical Applications. SLAS Technol. 2019, 24, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Zeng, G.; He, Z.; Mao, L.; Liu, M.; Huang, H.; Deng, F.; Zhang, X.; Wei, Y. Fabrication and biomedical applications of AIE active nanotheranostics through the combination of a ring-opening reaction and formation of dynamic hydrazones. J. Mater. Chem. B 2016, 4, 5692–5699. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Meng, J.; Li, C.; Wang, X.; Ye, Y.; Sun, K. Update on the Synthesis of N-Heterocycles via Cyclization of Hydrazones (2017–2021). Adv. Synth. Catal. 2021, 363, 5235–5265. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Russ. Molecules 2021, 26, 5705. [Google Scholar] [CrossRef]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Rodrigues, E.G.; Amorim Reis, A.K.C.; Longo, L.S.; Ogata, F.T.; Moretti, A.I.S.; da Costa, P.E.; Teodoro, A.C.S.; Toledo, M.S.; Stern, A. Nitric oxide and interactions with reactive oxygen species in the development of melanoma, breast, and colon cancer: A redox signaling perspective. Nitric Oxide 2019, 89, 1–13. [Google Scholar] [CrossRef]
- Cui, Q.; Yang, Y.; Ji, N.; Wang, J.-Q.; Ren, L.; Yang, D.-H.; Chen, Z.-S. Gaseous signaling molecules and their application in resistant cancer treatment: From invisible to visible. Future Med. Chem. 2019, 11, 323–336. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.C.; Chen, X. Stimuli-Responsive NO Release for On-Demand Gas-Sensitized Synergistic Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 8383–8394. [Google Scholar] [CrossRef] [PubMed]
- Serafim, R.A.M.; Gonçalves, J.E.; de Souza, F.P.; de Melo Loureiro, A.P.; Storpirtis, S.; Krogh, R.; Andricopulo, A.D.; Dias, L.C.; Ferreira, E.I. Design, synthesis and biological evaluation of hybrid bioisoster derivatives of N-acylhydrazone and furoxan groups with potential and selective anti-Trypanosoma cruzi activity. Eur. J. Med. Chem. 2014, 82, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P.; Rojas, R.; Gilman, R.H.; Sauvain, M.; Lima, L.M.; Barreiro, E.J.; González, M.; Cerecetto, H. Hybrid furoxanyl N-acylhydrazone derivatives as hits for the development of neglected diseases drug candidates. Eur. J. Med. Chem. 2013, 59, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, S.; Cortese, D.; Vottero, F.; Rolando, B.; Kommer, V.P.; Williams, D.L.; Fruttero, R.; Gasco, A. New praziquantel derivatives containing NO-donor furoxans and related furazans as active agents against Schistosoma mansoni. Eur. J. Med. Chem. 2014, 84, 135–145. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, G.F.; de Souza, P.C.; Marino, L.B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M.C.; Pavan, F.R.; dos Santos, J.L. Synthesis and biological activity of furoxan derivatives against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2016, 123, 523–531. [Google Scholar] [CrossRef]
- Larin, A.A.; Fershtat, L.L.; Ustyuzhanina, N.E.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New hybrid furoxan structures with antiaggregant activity. Mendeleev Commun. 2018, 28, 595–597. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant activity of water-soluble furoxans. Mendeleev Commun. 2018, 28, 49–51. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Ustyuzhanina, N.E.; Fershtat, L.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant effects of (1,2,5-oxadiazolyl)azasydnone ring assemblies as novel antiplatelet agents. Chem. Biol. Drug Des. 2021, in press. [Google Scholar] [CrossRef]
- Wang, C.; Xi, D.; Wang, H.; Niu, Y.; Liang, L.; Xu, F.; Peng, Y.; Xu, P. Hybrids of MEK inhibitor and NO donor as multitarget antitumor drugs. Eur. J. Med. Chem. 2020, 196, 112271. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Li, H.; Wang, K.; Wan, Q.; Li, J.; Zhou, Y.; Chen, Y. Novel Nitric Oxide Donors of Phenylsulfonylfuroxan and 3-Benzyl Coumarin Derivatives as Potent Antitumor Agents. ACS Med. Chem. Lett. 2018, 9, 502–506. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Polkovnichenko, M.S.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Novel Arylazo-1,2,5-oxadiazole Photoswitches: Synthesis, Photoisomerization and Nitric Oxide Releasing Properties. ChemPhotoChem 2020, 4, 5346–5354. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, G.F.; Pavan, A.R.; dos Santos, J.L. Heterocyclic N-oxides—A Promising Class of Agents against Tuberculosis, Malaria and Neglected Tropical Diseases. Curr. Pharm. Des. 2018, 24, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Bystrov, D.M.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Direct Synthesis of N-(1,2,5-Oxadiazolyl)hydrazones through a Diazotization/Reduction/Condensation Cascade. J. Org. Chem. 2020, 85, 15466–15475. [Google Scholar] [CrossRef] [PubMed]
- Fershtat, L.L.; Epishina, M.A.; Ovchinnikov, I.V.; Kachala, V.V.; Makhova, N.N. An effective synthesis of (1H-1,2,4-triazol-3-yl)furoxans. Chem. Heterocycl. Compd. 2015, 51, 754–759. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Makhova, N.N. Reactions of bromoacetyl derivatives of furoxan and furazan with S-nucleophiles. Russ. Chem. Bull. 1998, 47, 139–143. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).