SNAr Reactions on 2-Amino-4,6-dichloropyrimidine-5-carbaldehyde
Abstract
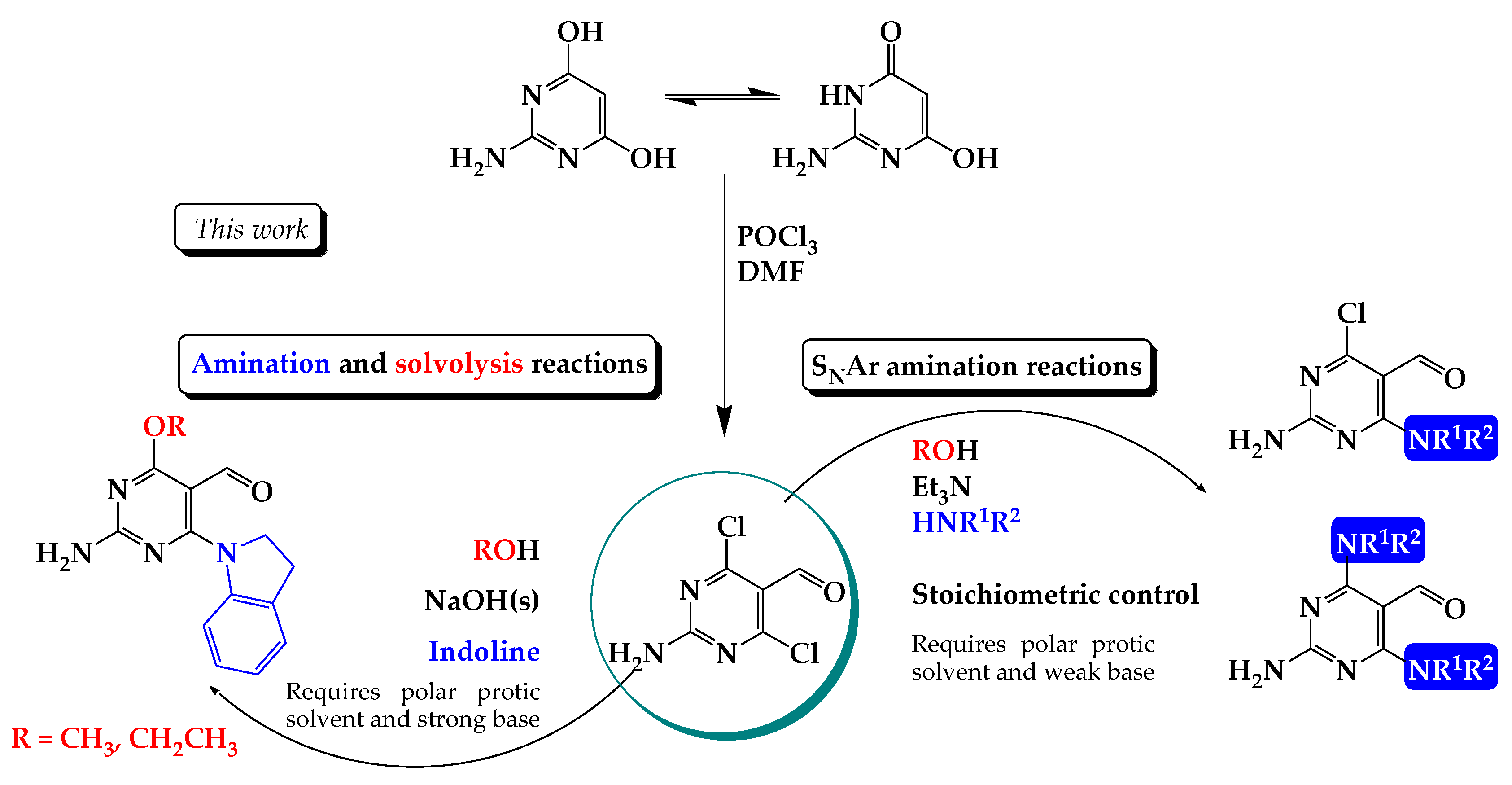
:1. Introduction
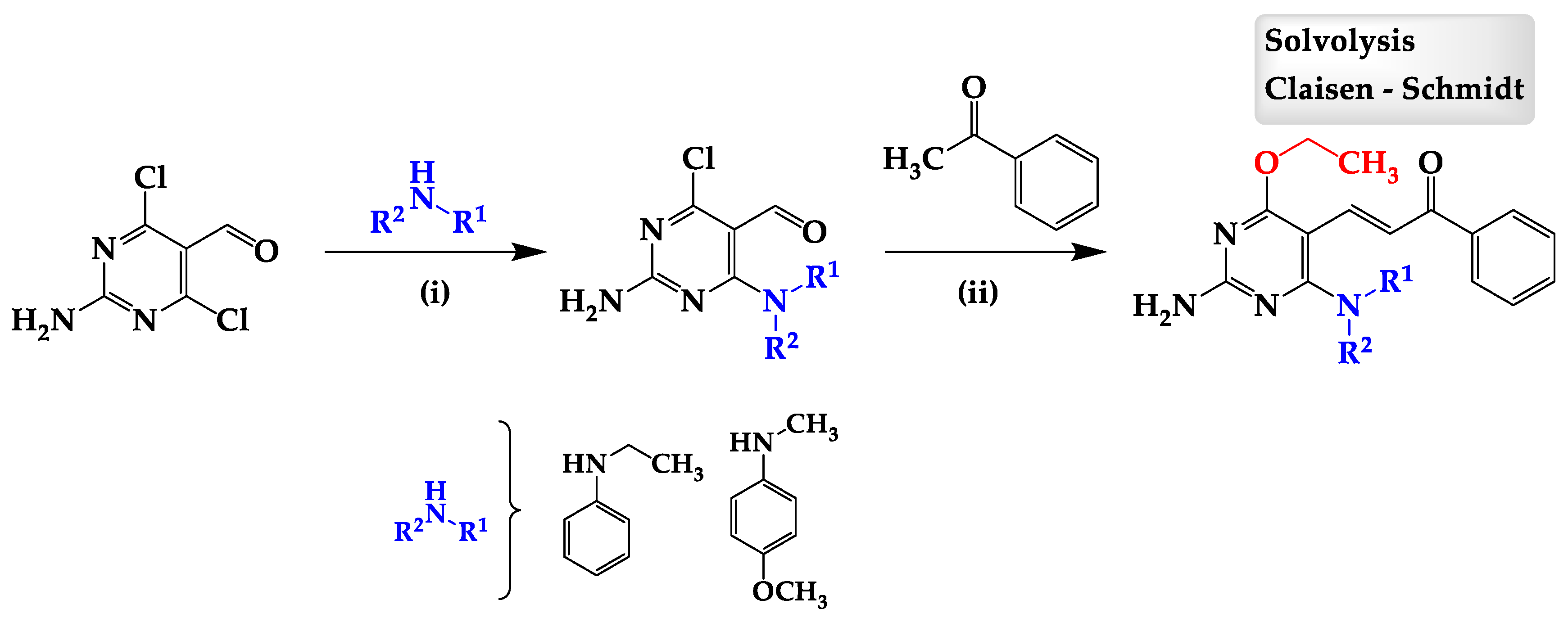
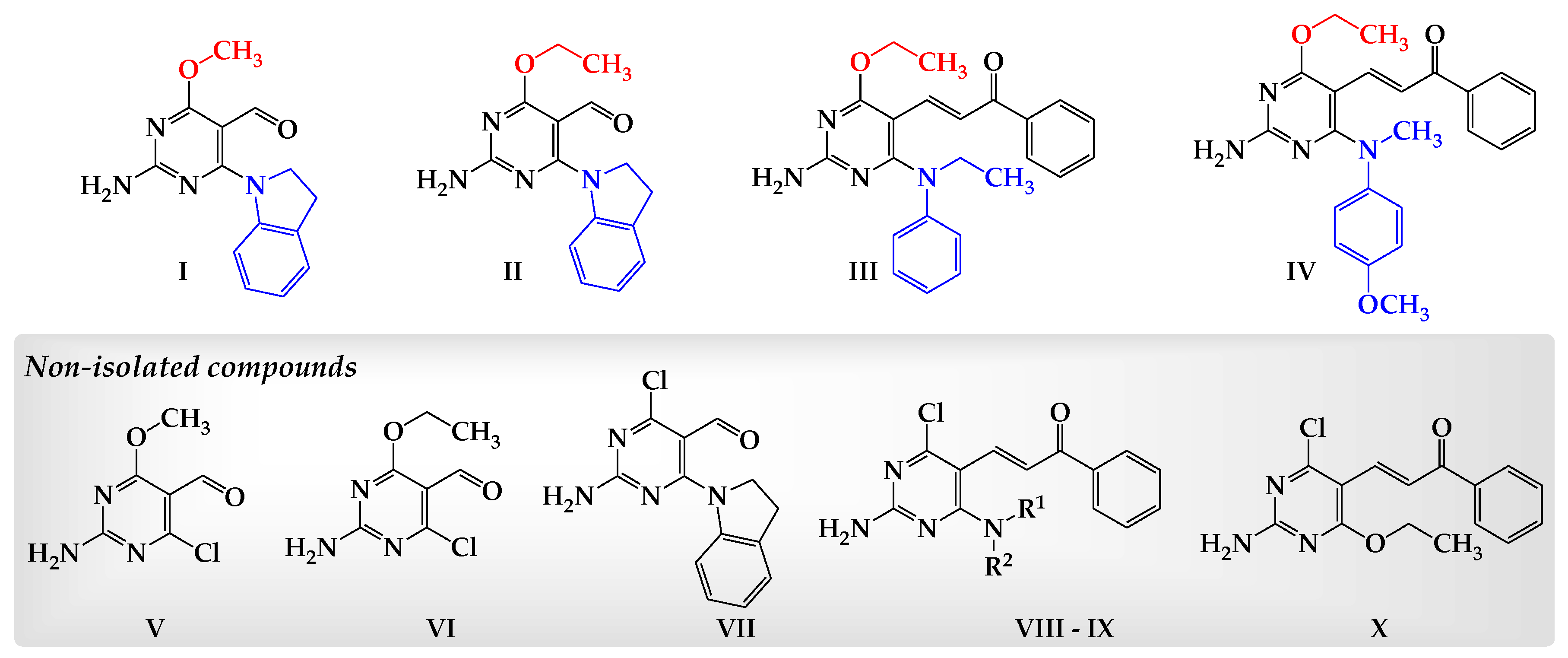
2. Results
Chemistry
3. Discussion
4. Materials and Methods
4.1. General Remarks
4.2. Synthesis and Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, É.; Szőllősi, G. Nitrogen-Containing Heterocycles as Significant Molecular Scaffolds for Medicinal and Other Applications. Molecules 2021, 26, 4617. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Gummidi, L.; Maddila, S.; Kumar Gangu, K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Madia, V.N.; Nicolai, A.; Messore, A.; De Leo, A.; Ialongo, D.; Tudino, V.; Saccoliti, F.; De Vita, D.; Scipione, L.; Artico, M.; et al. Design, Synthesis and Biological Evaluation of New Pyrimidine Derivatives as Anticancer Agents. Molecules 2021, 26, 771. [Google Scholar] [CrossRef] [PubMed]
- Dolšak, A.; Mrgole, K.; Sova, M. Microwave-Assisted Regioselective Suzuki Coupling of 2,4-Dichloropyrimidines with Aryl and Heteroaryl Boronic Acids. Catalysts 2021, 11, 439. [Google Scholar] [CrossRef]
- Kharlamova, A.D.; Abel, A.S.; Averin, A.D.; Maloshitskaya, O.A.; Roznyatovskiy, V.A.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. Mono- and Diamination of 4,6-Dichloropyrimidine, 2,6-Dichloropyrazine and 1,3-Dichloroisoquinoline with Adamantane-Containing Amines. Molecules 2021, 26, 1910. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.; Sankhe, K.; Bhandare, R.R.; Edis, Z.; Bloukh, S.H.; Khan, T.A. Synthetic Strategies of Pyrimidine-Based Scaffolds as Aurora Kinase and Polo-like Kinase Inhibitors. Molecules 2021, 26, 5170. [Google Scholar] [CrossRef]
- Drewry, D.H.; Annor-Gyamfi, J.K.; Wells, C.I.; Pickett, J.E.; Dederer, V.; Preuss, F.; Mathea, S.; Axtman, A.D. Identification of pyrimidine-based lead compounds for understudied kinases implicated in driving neurodegeneration. J. Med. Chem. 2022, 65, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Jansa, P.; Holý, A.; Draĉínnský, M.; Kolman, V.; Janeba, Z.; Kostecká, P.; Kmoníĉková, E.; Zídek, Z. 5-Substituted 2-amino-4,6-dihydroxypyrimidines and 2-amino-4,6-dichloropyrimidines: Synthesis and inhibitory effects on immune-activated nitric oxide production. Med. Chem. Res. 2014, 23, 4482–4490. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J.; de la Torre, J.M. 4-Aminopyrimidine-5-carbaldehydes as intermediates in a Friedländer type synthesis of 7-arylpyrido[2,3-d]pyrimidines. ARKIVOC Online J. Org. Chem. 2009, 9–27. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Gu, M.; Ma, H.; Tang, J.; Lu, W.; Nan, F.-J. An Efficient Synthesis of 2-Chloropyrimidines via Pd-catalyzed Regioselective Dechlorination of 2,4-Dichloropyrimidines in the Presence of NaHCO3. Chin. J. Chem. 2008, 26, 962–964. [Google Scholar] [CrossRef]
- Mittersteiner, M.; Farias, F.F.S.; Bonacorso, H.G.; Martins, M.A.P.; Zanatta, N. Ultrasound-assisted synthesis of pyrimidines and their fused derivatives: A review. Ultrason. Sonochem. 2021, 79, 105683. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Biginelli Reaction Mediated Synthesis of Antimicrobial Pyrimidine Derivatives and Their Therapeutic Properties. Molecules 2021, 26, 6022. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J. Delia. Grignard Reactions Involving Halogenated Pyrimidines. J. Heterocycl. Chem. 2013, 50, 735–745. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Padusha, M.S.A.; Sajith, A.M. Application of Palladium Based Precatalytic Systems in the Suzuki-Miyaura Cross-Coupling Reactions of Chloro- Heterocycles. ChemistrySelect 2020, 5, 9005–9016. [Google Scholar] [CrossRef]
- Malik, A.; Rasool, N.; Kanwal, I.; Hashmi, M.A.; Zahoor, A.F.; Ahmad, G.; Altaf, A.A.; Shah, S.A.A.; Sultan, S.; Zakaria, Z.A. Suzuki–miyaura reactions of (4-bromophenyl)-4,6-dichloropyrimidine through commercially available palladium catalyst: Synthesis, optimization and their structural aspects identification through computational studies. Processes 2020, 8, 1341. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.S.; Min, S.-J. Facile Synthesis of 2-Amino-4-alkoxypyrimidines via Consecutive Nucleophilic Aromatic Substitution (SNAr) Reactions. Bull. Korean Chem. Soc. 2016, 37, 1998–2008. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.J.; Londhe, A.M.; Pae, A.N.; Choo, H.; Kim, H.J.; Min, S.-J. Synthesis and Biological Evaluation of Disubstituted Pyrimidines as Selective 5-HT2C Agonists. Molecules 2019, 24, 3234. [Google Scholar] [CrossRef]
- Bruening, F.; Lovelle, L.E. Highly Regioselective Organocatalytic SNAr Amination of 2,4-Dichloropyrimidine and Related Heteroaryl Chlorides. Eur. J. Org. Chem. 2017, 2017, 3222–3228. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Marchal, A.; Cobo, J. Microwave-assisted synthesis of pyrazolo[3,4-d]pyrimidines from 2-amino-4,6-dichloropyrimidine-5-carbaldehyde under solvent-free conditions. Tetrahedron Lett. 2008, 49, 3257–3259. [Google Scholar] [CrossRef]
- Yadav, G.D.; Wagh, D.P. Claisen-Schmidt Condensation using Green Catalytic Processes: A Critical Review. ChemistrySelect 2020, 5, 9059–9085. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trilleras, J.; Pérez-Gamboa, A.; Quiroga, J. SNAr Reactions on 2-Amino-4,6-dichloropyrimidine-5-carbaldehyde. Molbank 2022, 2022, M1426. https://doi.org/10.3390/M1426
Trilleras J, Pérez-Gamboa A, Quiroga J. SNAr Reactions on 2-Amino-4,6-dichloropyrimidine-5-carbaldehyde. Molbank. 2022; 2022(3):M1426. https://doi.org/10.3390/M1426
Chicago/Turabian StyleTrilleras, Jorge, Alfredo Pérez-Gamboa, and Jairo Quiroga. 2022. "SNAr Reactions on 2-Amino-4,6-dichloropyrimidine-5-carbaldehyde" Molbank 2022, no. 3: M1426. https://doi.org/10.3390/M1426
APA StyleTrilleras, J., Pérez-Gamboa, A., & Quiroga, J. (2022). SNAr Reactions on 2-Amino-4,6-dichloropyrimidine-5-carbaldehyde. Molbank, 2022(3), M1426. https://doi.org/10.3390/M1426







