Abstract
N-(2,2-Diphenylethyl)-2-(6-methoxynaphthalen-2-yl)propanamide was prepared by a reaction between 2,2-diphenylethan-1-amine and naproxen in high yield. The newly obtained naproxen derivative was fully analyzed and characterized via 1H, 13C, UV, IR, and mass spectral data.
1. Introduction
Naproxen 1 (Figure 1) is part of the aryl propionic family of non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs are commonly used worldwide. They can be either selective or non-selective cyclooxygenase (COX) inhibitors. Profens are considered one of the most important groups of non-selective COX inhibitors used in the treatment of inflammation associated with tissue injury [1].
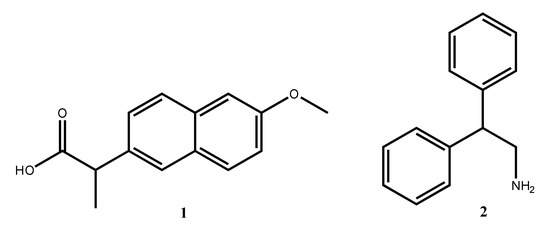
Figure 1.
Structural formulas of naproxen and 2,2-diphenylethan-1-amine.
2,2-diphenylethylamine 2 (Figure 1) is a fragment present in the structure of many naturally occurring compounds. Cherylline, latifine, and nomifensine (Figure 2) are representatives of alkaloids that contain 2,2-diphenylethan-1-amine in their structures. They represent very rare natural 4-aryl-1,2,3,4-tetrahydroisoquinoline alkaloids which are of interest because of their broad spectrum of biological activities. Representatives of 4-aryl-1,2,3,4-tetrahydroisoquinoline alkaloids exhibit broad spectrum of biological activities as: antagonistic [2], antibacterial [3], antiplasmodial [4], estrogen agonist/antagonist [5], and serotonin (5-HT) re-uptake [6].
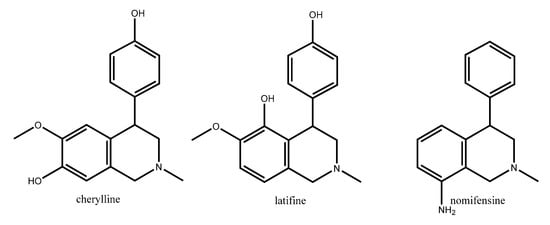
Figure 2.
Structural formulas of alkaloids containing 2,2-diphenylethan-1-amine as a fragment.
Due to the importance of amides in the pharmaceutical synthesis [7,8], a coupling between naproxen and 2,2-diphenylethylamine via amide bond formation was achieved in order to obtain N-(2,2-diphenylethyl)-2-(6-methoxynaphthalen-2-yl)propanamide 3.
2. Results
Herein, we report the successfully synthesized N-(2,2-diphenylethyl)-2-(6-methoxynaphthalen-2-yl)propanamide 3, as shown in Scheme 1.
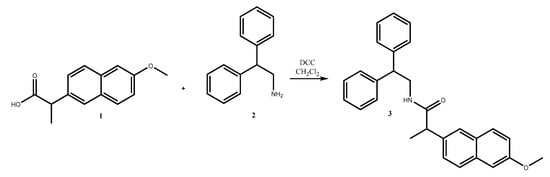
Scheme 1.
Synthesis of N-(2,2-diphenylethyl)-2-(6-methoxynaphthalen-2-yl)propanamide 3.
An easy and handy synthetic procedure for amide synthesis is the N,N′-dicyclohexylcarbodiimide (DCC)-mediated coupling between carboxylic acids and amines. DCC is used for the preparation of esters, amides, or anhydrides. DCC reacts with the carboxyl group of naproxen to produce an activated acylating agent that reacts with the amino group of the other molecule to form an amide bond.
The resultant compound is characterized by its melting point, 1H and 13C-NMR, UV, IR, and HRMS spectra.
3. Materials and Methods
All reagents and chemicals were purchased from commercial sources (Sigma-Aldrich S.A. and Riedel-de Haën, Sofia, Bulgaria) and used as received. Melting points were determined on a Boetius hot stage apparatus and are uncorrected. The NMR spectral data were recorded on a Bruker Avance II +600 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA). 1H-NMR and 13C-NMR spectra for compound 3 were taken in DMSO-d6 at 600 MHz and at 150.9 MHz, respectively. Chemical shifts are given in relative ppm and were referenced to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard; the coupling constants are indicated in Hz. The NMR spectra were recorded at room temperature (ac. 295 K). Mass analyses were carried out on a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany).
Synthesis of N-(2,2-Diphenylethyl)-2-(6-methoxynaphthalen-2-yl)propanamide 3
N,N’-Dicyclohexylcarbodiimide (1 mmol, 0.206 g) was added to a solution of naproxen (1 mmol, 0.230 g) in CH2Cl2. The reaction mixture was stirred at room temperature for 10 min. After the addition of 2,2-diphenylethylamine (1 mmol, 0.197 g), the reaction mixture was stirred for 50 min and the formation of white crystalline dicyclohexylurea was observed and then separated by filtration over a sintered glass filter. The filtrate was washed with a diluted hydrochloric acid, a saturated solution of Na2CO3, and brine. The combined organic layers were dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure. The compound was purified by column chromatography (silica gel 60, 70–230 mesh, Merck; diethyl ether: petroleum ether = 1:1 (v/v).
N-(2,2-Diphenylethyl)-2-(6-methoxynaphthalen-2-yl)propanamide (3): white solid (m.p. 110–112 °C), yield 89% (0.364 g), 1H-NMR (600 MHz, DMSO) δ 8.03 (t, J = 5.7 Hz, 1H), 7.72 (d, J = 9.0 Hz, 1H), 7.67 (d, J = 8.5 Hz, 1H), 7.59 (s, 1H), 7.27–7.25 (m, 2H), 7.23 (d, J = 4.4 Hz, 4H), 7.20–7.12 (m, 7H), 4.15 (t, J = 7.9 Hz, 1H), 3.86 (s, 3H), 3.74–3.68 (m, 1H), 3.67–3.60 (m, 2H), 1.30 (d, J = 7.0 Hz, 3H). 13C-NMR (151 MHz, DMSO) δ 173.88 (C=O), 157.39 (Ar, C-O-CH3), 143.30 (C, Ar), 137.63(C, Ar), 133.53 (C, Ar), 129.57 (C, Ar), 128.81 (C, Ar), 128.73 (C, Ar), 128.39 (C, Ar), 128.33 (C, Ar), 126.93 (C, Ar), 126.73 (C, Ar), 125.66 (C, Ar), 118.93 (C, Ar), 106.07 (C, Ar), 55.59 (-OCH3), 50.34 ((Ph)2CH), 45.18 (CH2), 43.73 (CH), 18.70 (CH-CH3). UV λmax, MeOH: 241 (ε = 12,500) nm. HRMS Electrospray ionization (ESI) m/z calcd for C28H28NO2+ = 410.2115, found 410.2119 (mass error Δm = 0.98 ppm). IR(KBr) νmax, cm−1: 3364 ν(N-H), 3058, 3033 ν(Csp2-H), 2996 νas(Csp3-H, >CH2), 2882 νs(Csp3-H, > CH2), 1655 ν(C=O), 1603 ν(C=C, Ph), 1533 δ(N-H) + ν(C-N), 1503 ν(C=C, Ph), 1481 δs(>CH2) and ν(C=C, Ph), 1457 ν(C=C, Ph), δas(CH3), δ(N-CH2), 1446, 1391, 1362 δs(CH3), 1342 δs(-CH<), 1271, 1260 ν(Ph-NH), 1228 ν(HN-C=O), 1208, 1189, 1162 ν(C-N), 1121, 1090, 1067, 1052, 1028, 928, 893, 853 γ(Csp2-H), 816 γ(Csp2-H), 755, 732 γ(Csp2-H), 696, 670, 640, 583, 473, 428 δ(C-N-C).
Copies of all spectra and ESI-HRMS (Figures S1–S5) are provided in the Supplementary Materials file.
Supplementary Materials
Supplementary materials are available online. Figure S1: 1H-NMR spectrum of compound 3, Figure S2: 13C-NMR spectrum of compound 3, Figure S3: UV spectrum of compound 3, Figure S4: ESI-HRMS of compound 3, Figure S5: IR spectrum of compound 3.
Author Contributions
The first two authors (S.M. and I.I.) are responsible for the synthesis, writing, revising, NMR, IR analysis and final English check of the manuscript. The third author (D.B.) is responsible for the UV and ESI-HRMS analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Fund of the Bulgarian Ministry of Education and Science, grant number KП 06 M29/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and supporting Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pereva, S.; Sarafska, T.; Petrov, V.; Angelova, S.; Spassov, T. Inclusion complexes of (S)-naproxen and native cyclodextrins: Supramolecular structure and stability. J. Mol. Struct. 2021, 1235, 130218. [Google Scholar] [CrossRef]
- Dong, H.; Sheng, J.Z.; Lee, C.M.; Wong, T.M. Calcium antagonistic and antiarrhythmic actions of CPU-23, a substituted tetrahydroisoquinoline. Br. J. Pharmacol. 1993, 109, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernan, V.S.; Montenegro, D.A.; Korshalla, J.D.; Maiese, W.M.; Steinberg, D.A.; Greenstein, M. Bioxalomycins new antibiotics produced by the marine Streptomyces spp. LL-31F508: Taxonomy and fermentation. J. Antibiot. 1994, 47, 1417–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, R.M.; Nichols, D.E.; Foreman, M.M.; Truex, L.L. Evaluation of isomeric 4-(chlorohydroxyphenyl)-1,2,3,4-tetrahydroisoquinolines as dopamine D-1 antagonists. J. Med. Chem. 1987, 30, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Chesworth, R.; Zawistoski, M.P.; Lefker, B.A.; Cameron, K.O.; Day, R.F.; Mangano, F.M.; Rosati, R.L.; Colella, S.; Petersen, D.N.; Brault, A.; et al. Tetrahydroisoquinolines as subtype selective estrogen agonists/antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.M.; Barbeir, A.J.; Wilson, S.J.; Miller, K.; Boggs, J.D.; Fraser, I.C.; Mazur, C.; Lovenberg, T.W.; Carruthers, N.I. Dual serotonin transporter inhibitor/histamine H3 antagonists: Development of rigidified H3 pharmacophores. Bioorg. Med. Chem. Lett. 2007, 17, 5325–5329. [Google Scholar] [CrossRef] [PubMed]
- Trabocchi, A.; Mannino, C.; Machetti, F.; Bernardis, D.F.; Arancia, S.; Cauda, R.; Cassone, A.; Guarna, A. Identification of inhibitors of drug-resistant Candida albicans strains from a library of bicyclic peptidomimetic compounds. J. Med. Chem. 2010, 53, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Rees, D.; Rankovic, Z.; Morphy, R. Synthesis of tertiary amines using a polystyrene (REM) resin. J. Am. Chem. Soc. 1997, 119, 3288–3295. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).