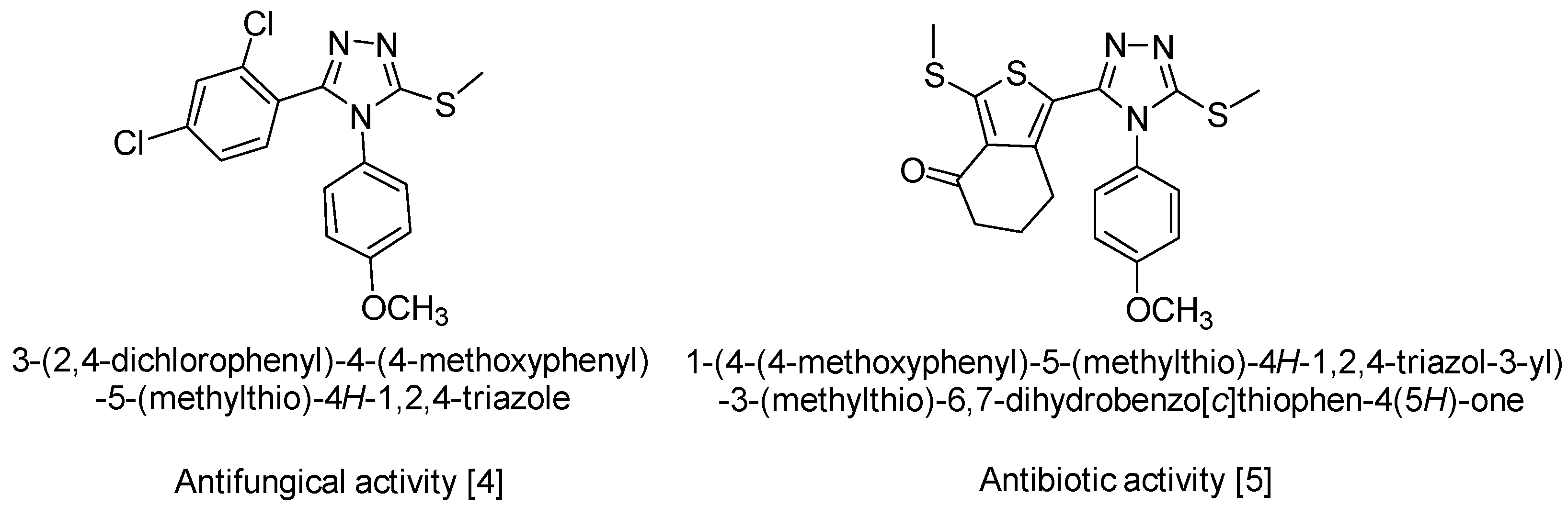Abstract
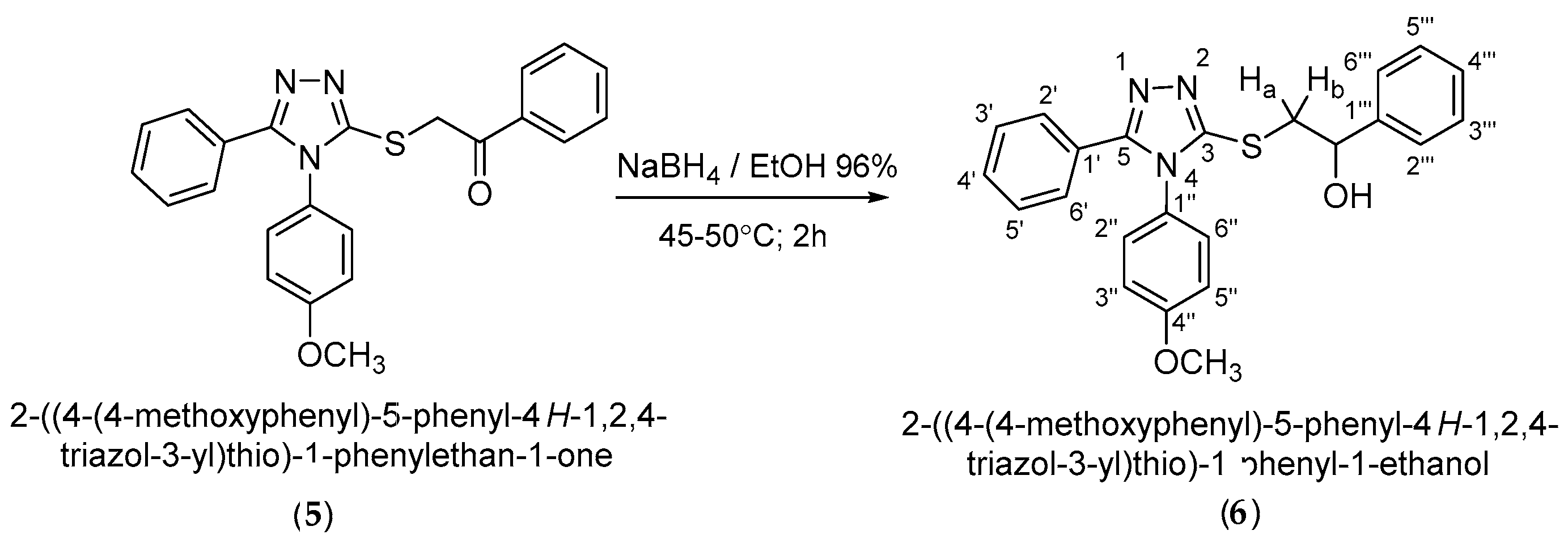
4-(4-Methoxyphenyl)-5-phenyl--4H-1,2,4-triazole-3-thiol (4) was alkylated to 2-{[4-(-4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5) in alkaline conditions using 2-bromo-1-phenylethanone. The alkylated compound (5) was reduced at the carbonyl group to the corresponding racemic secondary alcohol with an asymmetric carbon, (R,S)-2-{[4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (6). Both synthesized compounds, ketone (5) and secondary alcohol (6), are new and have not been reported yet in the literature. All the synthesized compounds were characterized by IR, 1D and 2D NMR 1H-1H, 1H-13C and 1H-15N-NMR spectroscopy and by elemental analysis.
1. Introduction
The recent literature reveals that the mercapto- and thione-substituted 1,2,4-triazole moieties should be an important structural feature of a wide range of synthetic medicines [1]. A variety of medicinal actions have been reported, ranging from anti-tubercular action [2] to protein inhibitory action involved in the mechanism of diseases such as diabetes, obesity and cancer [3]. Other S-alkylated compounds derived from 4H-1,2,4-triazole-3-thiol 4,5-disubstituted show proven antifungal [4], antimicrobial and antibiotic activity [5] (Scheme 1).
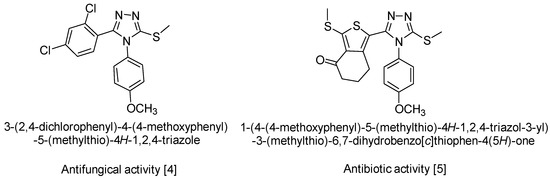
Scheme 1.
Biological activity of similar compounds.
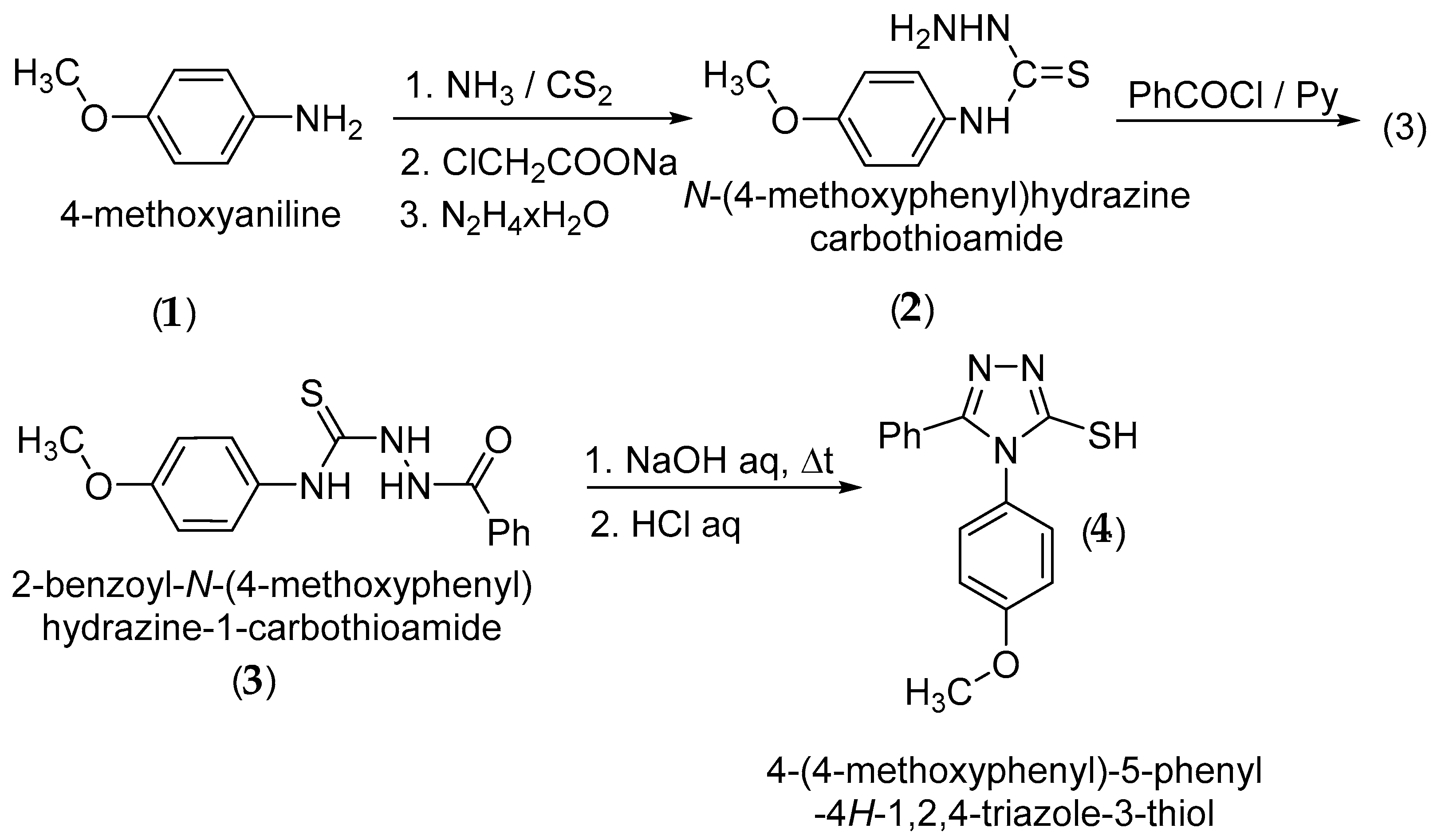
4-(4-Methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (4) was synthesized starting from 4-methoxyaniline (1) via the corresponding N-(4-methoxyphenyl)hydrazinecarbothioamide (2), followed by acylation to 2-benzoyl-N-(4-methoxyphenyl)hydrazine-1-carbothioamide (3) and cyclization of (3) to 4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (4) according to the literature methods (Scheme 2) [6,7,8,9,10]. The S-alkylation was performed using cesium carbonate as an alkaline base [11,12] and the reduction of the ketone group to the corresponding secondary alcohol was carried out with sodium borohydride [13].
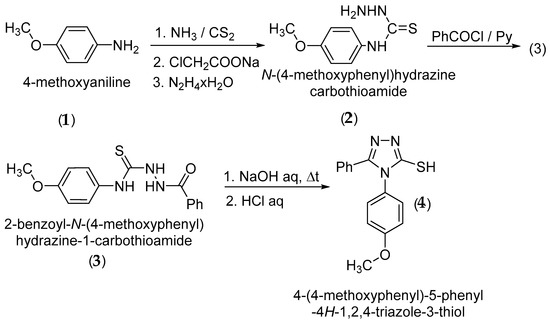
Scheme 2.
Synthetic route to 4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol.
2. Results and Discussion
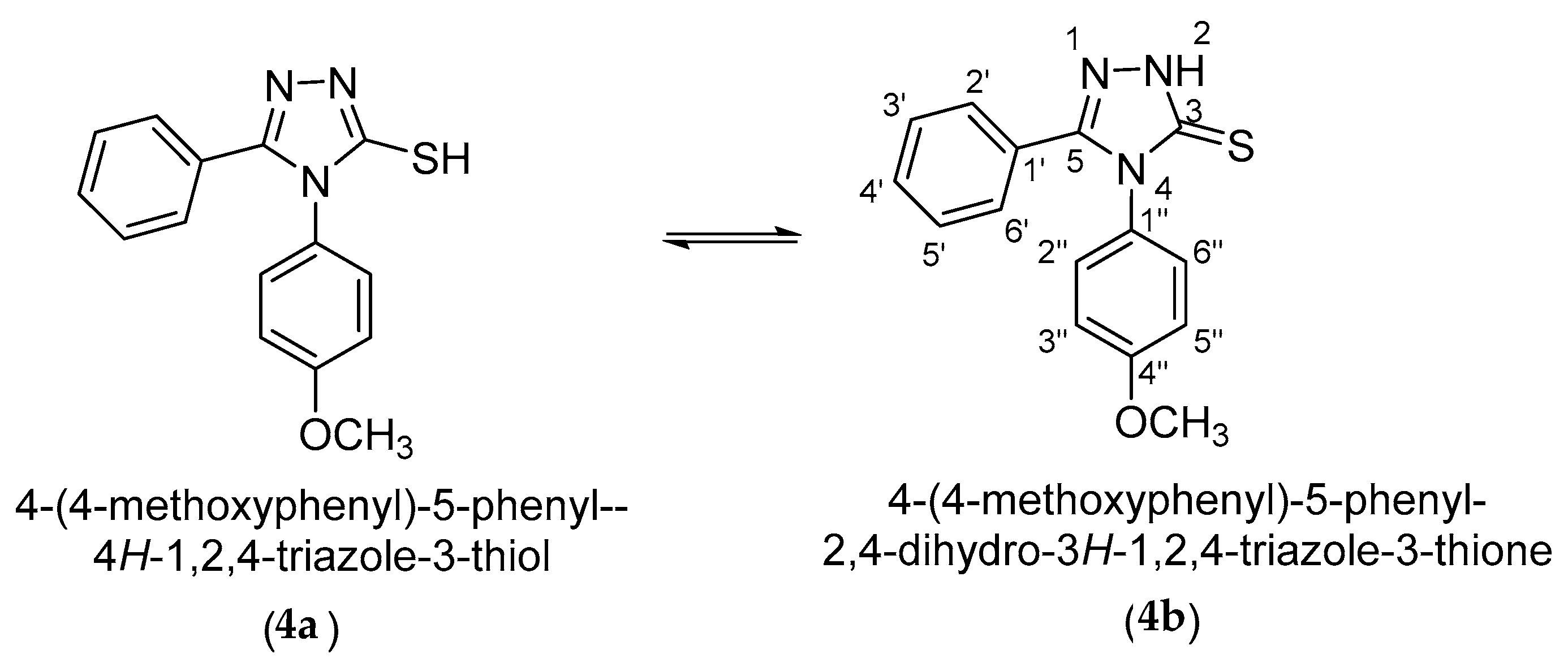
3-Mercaptotriazole (4) can theoretically have two tautomeric forms: the thiol form (4a) and the thione form (4b). As a result, alkylation in a basic medium can theoretically occur as S-alkylation at the tautomeric form (4a) or as N-alkylation at the tautomeric form (4b) (Scheme 3).
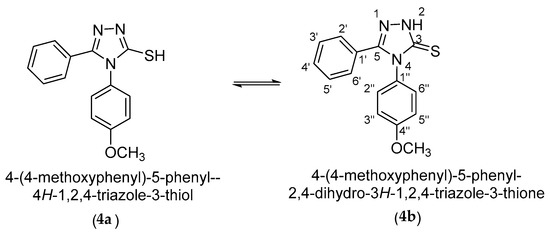
Scheme 3.
Tautomeric equilibrium of the 4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (4).
From the 1H and 13C-NMR spectra, it is confirmed that the tautomeric equilibrium is completely shifted to the tautomeric form (4b) in Py-d5. This shift of equilibrium is confirmed by the deshielded signal of the 2-N-H proton at 16.00 ppm, as well as by the deshielded signal of the 3-C carbon atom at 171.7 ppm, corresponding to a C=S thionic carbon atom.
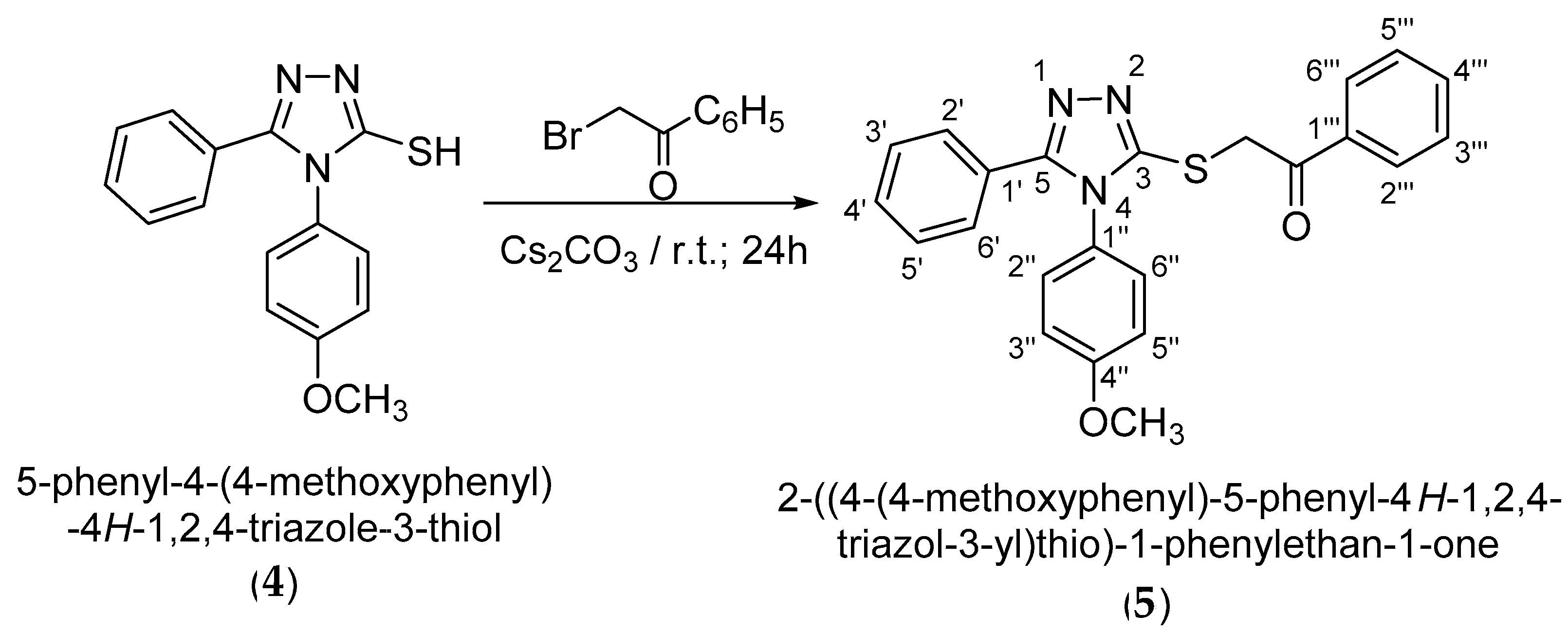
Following alkylation using cesium carbonate as a base in N,N-dimethylformamide, it has been observed that the alkylation occurs exclusively at the thiol group as S-alkylation. This is observed from 2D NMR spectroscopic analysis by analyzing the couplings over two to three bonds in the HMBC spectrum as well as by the shifting of the signal of the triazolic carbon 3-C atom to a lower δ value at 151.7 ppm, corresponding to a thiol type carbon atom. S-Alkylation of 4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (4) was carried out using 2-bromo-1-phenylethanone in DMF in the presence of Cs2CO3 at room temperature. 2-{[4-(-4-Methoxyphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5) resulting from S-alkylation was obtained in a yield of 79% after recrystallization from ethanol (Scheme 4).
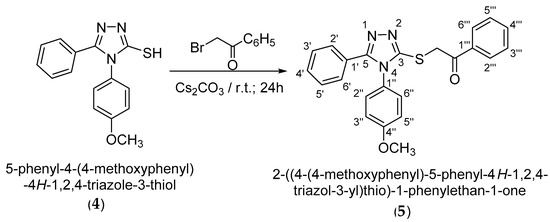
Scheme 4.
Synthetic route to 2-{[4-(-4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-yl]thio}-1-phenylethan-1-one.
Reduction of the carbonyl group in the ketone (5) to the racemic secondary alcohol (R,S)-2-{[4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (6) was accomplished with sodium borohydride in ethanol 96%. Secondary alcohol (6) was obtained in a yield of 77.0% after recrystallization from ethanol (Scheme 5).
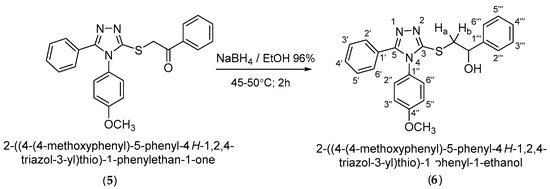
Scheme 5.
Synthetic route to 2-{[4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-yl]thio}-1-phenyl-1-ethanol.
From the correlative 1H-15N HMBC spectra, the signal for the 4-N nitrogen atom in all the synthesized compounds could be identified by its coupling over three bonds with hydrogen atoms in the ortho positions of the phenyl ring attached to this atom. This long-range coupling was very useful in the assignment of the corresponding 1H-NMR signals for the ortho protons on the phenyl ring bound to the 4-N nitrogen atom.
The methylene protons in the obtained secondary alcohol (5) are diastereotopic and appear in the 1H-NMR spectrum at different δ values as two distinct doublets of doublets. This is specific for a methylene group attached to an asymmetric carbon atom. From the 1H-13C HMBC spectrum, the long-range coupling over three bonds of the methylene diastereotopic protons with the 3-C triazole carbon atom is observed, thus further confirming the S-alkylation.
3. Materials and Methods
The chemical reagents were purchased from commercial sources and used in syntheses with no further purification. Melting points were determined on a Böetius PHMK (Veb Analytik Dresden, Dresden, Germany) melting point apparatus and are uncorrected. Infrared spectra (IR) were recorded as KBr disks on a Jasco FT/IR-410 spectrometer (JASCO Corporation, Tokyo, Japan). NMR spectra were recorded on a Bruker AVANCE III 500 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany), in DMSO-d6 and Py-d5 using TMS as an internal standard for protons and carbons. Chemical shifts are reported in ppm units and the coupling constants are given in Hz.
3.1. NMR Characterization of 4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (4)
1H-NMR (Py-d5, 500 MHz): δ (ppm): 16.00 (s, 1H, -NH), 7.64-7.62 (m, 2H, 2′-H, 6′-H), 7.50 (d, 2H, J = 8.94 Hz, 2″-H, 6″-H), 7.38-7.31 (m, 3H, 3′-H, 4′-H, 5′-H), 7.03 (d, 2H, J = 8.94 Hz, 3″-H, 5″-H), 3.35 (s, 3H, -OCH3);
13C-NMR (Py-d5, 125 MHz): δ (ppm): 171.7 (C=S), 160.8 (4″-C), 151.9 (5-C), 131.0 (4′-C); 130.7 (2″-C, 6″-C), 129.4 (3′-C, 5′-C), 129.3 (2′-C, 6′-C), 128.8 (1″-C), 127.6 (1′-C), 115.5 (3″-C, 5″-C), 55.8 (O-CH3);
15N-NMR (Py-d5, 50 MHz) δ (ppm): 182.6 (4-N).
(All spectra are reported in Supplementary Materials)
3.2. Synthesis of 2-{[4-(-4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5)
4-(4-Methoxyphenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (4) (0.00125 moles, 0.35 g) was magnetically stirred with cesium carbonate (0.00138 mol, 0.45 g) dissolved in ethanol (0.351 moles, 20.00 mL), at room temperature. After the cesium salt was dissolved, a solution of 2-bromo-1-phenylethanone (0.0012 moles, 0.25 g) in ethanol (0.263 moles, 15.00 mL) was added in the reaction mixture. The resulting mixture was left to stir for 24 h and after that was precipitated in water. The solid formed was collected by filtration, washed with water, dried and recrystallized from EtOH. The ketone (5) was obtained in a yield of 79.0%.
m.p.: 150–152 °C.
IR (KBr, cm−1): 3062, 2975, 2900, 1672, 1589; 1510, 1446, 1386, 1253, 1189, 837, 756, 692.
1H-NMR (DMSO-d6, 500 MHz): δ (ppm): 8.04 (d, 2H, J = 8.26 Hz, 2‴-H, 6‴-H), 7.69 (t, 1H, J = 7.44 Hz, 4‴-H), 7.57 (t, 2H, J = 7.90 Hz, 3‴-H, 5‴-H), 7.41-7.34 (m, 7H, 2′-H, 3′-H, 4′-H, 5′-H, 6′-H, 2″-H, 6″-H), 7.08 (dt, 2H, J = 8.90 Hz, J = 2.05 Hz, 3″-H, 5″-H) 4.94 (s, 2H, -CH2); 3.82 (s, 3H, -OCH3);
13C-NMR (DMSO-d6, 125 MHz): δ (ppm): 193.0 (C=O), 159.9 (4″-C), 154.4 (5-C), 151,7 (3-C), 135.2 (1‴-C), 133.6 (4‴-C), 129.6 (4′-C), 128.8 (2″-C, 5″-C), 128.7 (3‴-C, 5‴-C); 128.5 (3′-C, 5′-C), 128.3 (2‴-C, 6‴-C), 127.7 (2′-C, 6′-C), 126.6 (1′-C); 126.1 (1″-C), 115.0 (3″-C, 5″-C), 55.4 (-OCH3), 40.0 (-CH2);
15N-NMR (DMSO-d6, 50 MHz): δ (ppm): 177.2 (4-N).
(All spectra are reported in Supplementary Materials)
Elemental analysis for C23H19N3O2S Calcd. (%): C, 68.81; H, 4.77; N, 10.47; S, 7.99
Found (%): C, 68.75; H, 4.69; N, 10.40; S, 7.90.
3.3. Synthesis of 2-{[4-(4-methoxyphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (6)
2-{[4-(-4-Methoxyphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5) (0.0005 moles, 0.223 g) was dissolved in ethanol (0.386 moles, 22.00 mL) and heated in a water bath until the complete dissolution of the ketone. Afterwards, sodium borohydride (0.0008 moles, 0.03 g) was added in five stages at certain time intervals. After the last portion, the conversion of the reaction was checked, and the resulting mixture was precipitated in water. The solid formed was collected by filtration, washed with water, dried and recrystallized from ethanol. The secondary alcohol (6) was obtained in a yield of 77.0%.
m.p.: 170–172 °C;
IR (KBr, cm−1): 3348, 3060, 2975, 2892, 2835, 1604, 1508, 1446, 1388, 1247, 1170, 831, 777, 700.
1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 7.40–7.30 (m, 11H, 2′-H, 3′-H, 4′-H, 5′-H, 6′-H, 2″-H, 6″-H, 2‴-H, 3‴-H, 5‴-H, 6‴-H), 7.26 (t, 1H, J = 7.06 Hz 4‴-H), 7.06 (d, 2H, J = 8.85 Hz, 3″-H, 5″-H), 5.80 (d, 1H, J = 4.78 Hz, -OH); 4.90-4.87 (m, 1H, -CH-OH), 3.80 (s, 3H, -OCH3), 3.54 (dd, 1H, J = 12.99 Hz, J = 4.48 Hz, Ha), 3.39-3.32 (m, 1H, Hb).
13C-NMR (DMSO-d6, 125 MHz) δ (ppm): 159.8 (4″-C), 154.3 (5-C), 152.7 (3-C), 143.7 (1‴-C), 129.5 (4′-C), 128.9 (2″-C, 6″-C), 128.4 (3′-C, 5′-C), 128.0 (3‴-C, 5‴-C), 127.7 (2′-C, 6-C), 127.2 (4‴-C), 126.7 (1′-C), 126.2 (1″-C), 125.8 (2‴-C, 6‴-C), 114.9 (3″-C, 5″-C), 70.9 (-CH), 55.4 (O-CH3), 40.5 (-CH2).
15N-NMR (DMSO-d6, 50 MHz) δ (ppm): 176.9 (4-N).
(All spectra are reported in Supplementary Materials)
Elemental analysis for C23H21N3O2S Calcd. (%): C, 68.46; H, 5.25; N, 10.41; S, 7.95.
Found (%): C, 68.40; H, 5.20; N, 10.30; S, 7.90.
Supplementary Materials
The following are available online, Figure S1. 1H-NMR spectrum of compound 4 in Py-d5; Figure S2. 13C-NMR spectrum of compound 4 in Py-d5; Figure S3. FT-IR spectrum of compound 5; Figure S4. 1H-NMR spectrum of compound 5 in DMSO-d6; Figure S5. 13C-NMR spectrum of compound 5 in DMSO-d6; Figure S6. COSY 1H-1H spectrum of compound 5 in DMSO-d6; Figure S7. 13C DEPT135 spectrum of compound 5 in DMSO-d6; Figure S8. HMBC 1H-13C spectrum of compound 5 in DMSO-d6; Figure S9. HMBC 1H-15N spectrum of compound 5 in DMSO-d6; Figure S10. HSQCCED 1H-13C spectrum of compound 5 in DMSO-d6; Figure S11. FT-IR spectrum of compound 6; Figure S12. 1H-NMR spectrum of compound 6 in DMSO-d6; Figure S13. 13C-NMR spectrum of compound 6 in DMSO-d6; Figure S14. COSY 1H-1H spectrum of compound 6 in DMSO-d6; Figure S15. 13C DEPT135 spectrum of compound 6 in DMSO-d6; Figure S16. HMBC 1H-13C spectrum of compound 6 in DMSO-d6; Figure S17. HMBC 1H-15N spectrum of compound 6 in DMSO-d6; Figure S18. HSQCCED 1H-13C spectrum of compound 6 in DMSO-d6.
Author Contributions
Designed the experiments, V.B.; performed the experiments, F.-G.W.; analyzed the spectral data, V.B.; wrote the manuscript, F.-G.W. and V.B.; supervision and funding acquisitions, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P2-2.1-PED-2019-3414, within PNCDI III.
Data Availability Statement
The data presented in this study are available within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the corresponding author.
References
- Ghanaat, J.; Khalilzadeh, M.A.; Zareyee, D. Molecular docking studies, biological evaluation and synthesis of novel 3-mercapto-1,2,4-triazole derivates. Mol. Div. 2020, 25, 223–232. [Google Scholar] [CrossRef] [PubMed]
- De, S.K.; Stebbins, J.L.; Chen, L.H.; Riel-Mehan, M.; Machleidt, T.; Dahl, R.; Pellecchia, M. Design, Synthesis, and Structure—Activity Relationship of Substrate Competitive, Selective and In Vivo Active Triazole and Thiadiazole Inhibitors of the c-Jun N-Terminal Kinase. J. Med. Chem. 2009, 52, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.M.; Inturi, B.; Pujar, G.V.; Purohit, M.N.; Vijaykumar, G.S. Design, synthesis and 3D-QSAR studies of new diphenylamine containing 1,2,4-triazoles as potential antitubercular agents. Eur. J. Med. Chem. 2014, 84, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.N.; Kataky, J.C.S.; Baruah, J.N. Synthesis and antibacterial activity of 1-(2,4-dichlorobenzoyl)-4-substituted thiosemicarbazides, 1,2,4-triazoles and their methyl derivatives. J. Heterocycl. Chem. 1984, 21, 1225–1229. [Google Scholar] [CrossRef]
- Tehranchian, S.; Akbarzadeh, T.; Fazeli, M.; Reza, M.; Jamalifar, H.; Shafiee, A. Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5H)ones. Bioorg. Med. Chem. Lett. 2005, 15, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Dimri, A.K.; Parmar, S.S. Synthesis of 3-aryl-4-oxothiazolin-2-yl(4-ethoxy-3-methoxy)phenyl hydrazones as possible anticonvulsants. J. Heterocycl. Chem. 1978, 15, 335–336. [Google Scholar] [CrossRef]
- Radl, S. Preparation of some pyrazole derivatives by extrusion of elemental sulfur from 1,3,4-thiadiazines. Collect. Czech Chem. Commun. 1992, 57, 656–659. [Google Scholar] [CrossRef]
- Nuțiu, M.; Bercean, V.; Birău, M. Synthesis of some 4-aryl-thiosemicarbazides. Ann. West Univ. Timiş. 1996, 5, 7–10. [Google Scholar]
- Golovlyova, S.M.; Moskvichev, Y.A.; Alov, E.M.; Kobylinsky, D.B.; Ermolaeva, V.V. Synthesis of novel five-membered nitrogen-containing heterocyclic compounds from derivatives of arylsulfonyl and arylthioacetic and propionic acids. Chem. Heterocycl. Comp. 2001, 37, 1102–1106. [Google Scholar] [CrossRef]
- Ledeți, I.; Bercean, V.; Alexa, A.; Foica, C.; Șuta, L.-M.; Dehelean, C.; Trandafirescu, C.; Muntean, D.; Licker, M.; Fuliaș, A. Preparation and antibacterial properties of substituted 1,2,4-triazoles. J. Chem. 2015, 2015, 879343. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Smith, R.A.; Nischwitz, A.K.; Gavin, T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3–TBAI. Tetrahedron Lett. 2005, 46, 8931–8935. [Google Scholar] [CrossRef]
- Varala, R.; Rao, K.S. Cesium salts in organic synthesis: A Review. Curr. Org. Chem. 2015, 19, 1242–1274. [Google Scholar] [CrossRef]
- Gómez, A.B.; Ahlsten, N.; Platero-Prats, A.E.; Martín-Matute, B. Synthesis of 4,5-disubstituted 2-aminothiazoles from α,β-unsaturated ketones: Preparation of 5-benzyl-4-methyl-2-aminothiazolium hydrochloride salt. Org. Synth. 2014, 91, 185–200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).