Abstract
A novel 1,2,4-triazin-6-one derivative (2) was synthesized by the reaction of the oxazolone derivative 1 with 4-nitrobenzoic acid hydrazide in the presence of sodium acetate and glacial acetic acid. The title compound 2 was characterized on basis of IR, 1H-NMR, 13C-NMR and mass spectral data.
1. Introduction
1,2,4-Triazine represents a class of heterocyclic compounds possessing significant biological activities which makes them targets for research in the field of medicine and agriculture [1,2,3]. 1,2,4-Triazines have gained considerable pharmacological interest due to their anticonvulsant [4,5], anticancer [6,7,8,9], antiprotozoal [10], anti-viral [11], anti-malarial [12], antibacterial [13,14,15] and antifungal effects [16,17,18]. In the field of agriculture, they showed effects such as insecticides, herbicides, plant growth regulators and they are deployed for enhancing crop yield [19,20,21].
2. Results and Discussion
The title compound 2 was synthesized by refluxing 4-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-phenyloxazol-5(4H)-one 1 (see supplementary material) with 4-nitrobenzoic acid hydrazide in glacial acetic acid in the presence of sodium acetate, as presented in Scheme 1. The utilized azlactone was synthesized by the Erlenmeyer-Plochl method as discussed earlier [22]. The acid hydrazide was prepared from the acid via esterification followed by hydrazinolysis with hydrazine hydrate. In the present reaction, the acid hydrazide acts as a nucleophile which attacks the carbonyl group of the oxazolone ring, followed by ring cleavage with concomitant cyclization to form the triazinone derivative 2 [5].
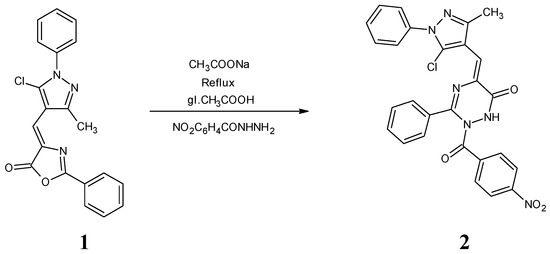
Scheme 1.
Synthetic route to the title compound.
3. Experimental
The melting point was determined in an open-end capillary tube on a digital melting point apparatus and is uncorrected. Infrared (IR) and proton nuclear magnetic resonance (1H-NMR) spectra were recorded on a Nicolet 380 FT-IR (KBr) and a Bruker DRX-300 instrument, respectively. Chemical shifts are expressed in ppm relative to TMS as an internal standard. The elemental analysis was performed on a Vario EL III CHN analyzer using sulphanilic acid as a standard. The ESI-MS spectrum was recorded on a Waters Micromass Q-TOF Micro. The homogeneity of the compounds was monitored by ascending thin-layer chromatography (TLC), visualized by iodine vapour.
Synthesis of 5-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-nitrobenzoyl)-3-phenyl-1, 2-dihydro-1, 2, 4-triazin-6(5H)-one (2)
An equimolar quantity (i.e., 0.01 mol) of compound 1 and of 4-nitrobenzoic acid hydrazide was refluxed along with sodium acetate (0.2 g) in glacial acetic acid (10 mL) for 6 h. The reaction mixture was then poured into crushed ice and stirred vigorously. The solid so obtained was filtered, washed with water, dried and recrystallised from ethanol.
Yield: 77%; m.p.: 242–244 °C; Rf: 0.75; mobile phase:toluene: ethyl acetate: formic acid (5:4:1); yellow crystalline solid.
IR (KBr) cm−1: 3273 (N-H), 3052 (aromatic C-H), 2933 (aliphatic C-H), 1719 (CONH), 1665 (C=O), 1609 (C=N, imine), 1576 (C=C), 697 (C-Cl).
1H-NMR (300 MHz, CDCl3): δ (ppm) 10.58 (s, 1H, CONH, D2O exchangeable), 8.17–8.15 (d, 2H, J = 8.7 Hz, Ar-H), 7.99–7.92 (m, 4H, Ar-H), 7.60–7.44 (m, 8H, Ar-H), 7.37 (s, 1H, CH=C), 2.82 (s, 3H, CH3).
13C-NMR (75 MHz, CDCl3): δ 170.3 (C=O, benzoyl), 164.3 (C6), 157.1 (C3), 151.5 (C-NO2), 150.2 (C-CH3) 133.8, 132.1, 131.6, 129.2, 129.0, 128.9, 128.1, 127.5, 125.0, 123.8, 122.7, 114.1, 16.6 (C-CH3).
Anal. Calcd. for C27H19ClN6O4: C, 61.54; H, 3.63; N, 15.94%; Found: C, 61.23; H, 3.89; N, 15.53%.
ESI-MS: m/z = 526.7 (M+), 528.7(M++2).
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
Authors are highly thankful to Rajendra Institute of Technology and Sciences, Sirsa for providing the necessary facilities for carrying out the research work.
References and Notes
- Boger, D.L. Diels-Alder cycloaddition reactions of heterocyclic azadienes: Scope and applications. Chem. Rev. 1986, 86, 781–793. [Google Scholar] [CrossRef]
- Boger, D.L. Diels-Alder reactions of azadienes. Tetrahedron 1983, 39, 2869–2939. [Google Scholar] [CrossRef]
- Neunhoeffer, H. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; p. 385. [Google Scholar]
- Yuen, A.W. Lamotrigine: A review of antiepileptic efficacy. Epilepsia 1994, 35, S33–S36. [Google Scholar] [CrossRef]
- Kaushik, D.; Khan, S.A.; Chawla, G. Design & synthesis of 2-(substituted aryloxy)-5-(substituted benzylidene)-3-phenyl-2, 5-dihydro-1H-[1, 2, 4] triazin-6-one as potential anticonvulsant agents. Eur. J. Med. Chem. 2010, 45, 3960–3969. [Google Scholar] [PubMed]
- Gibson, N.W.; Erickson, L.C.; Hickman, J.A. Effects of the antitumor agent 8-carbamoyl-3-(2-chloroethyl) imidazo [5, 1-d]-1, 2, 3, 5-tetrazin-4(3H)-one on the DNA of mouse L1210 cells. Cancer Res. 1984, 44, 1767–1771. [Google Scholar] [PubMed]
- Smith, R.H.; Scudiero, D.A.; Michejda, C.J. 1, 3-Dialkyl-3-acyltriazenes, a novel class of antineoplastic alkylating agents. J. Med. Chem. 1990, 33, 2579–2583. [Google Scholar] [CrossRef] [PubMed]
- Sztanke, K.; Rzymowska, J.; Niemczyk, M.; Dybała, I.; Kozioł, A.E. Synthesis, crystal structure and anticancer activity of novel derivatives of ethyl 1-(4-oxo-8-aryl-4,6,7,8 tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)formate. Eur. J. Med. Chem. 2006, 41, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sztanke, K.; Pasternak, K.; Sztanke, M.; Kandefer-Szerszen, M.; Kozioł, A.E.; Dybała, I. Crystal structure, antitumour and antimetastatic activities of disubstituted fused 1,2,4-triazinones. Bioorg. Med. Chem. Lett. 2009, 19, 5095–5100. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Husain, K.; Athar, F.; Azam, A. Synthesis and antiamoebic activity of 3, 7- dimethyl-pyrazolo [3, 4-e][1,2,4] triazin-4-yl thiosemicarbazide derivatives. Eur. J. Pharma. Sci. 2005, 25, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Saeda, M.; Fawzy, M.; El-Baz, M. Synthesis of some new 1, 6-dihydro-3-substituted 6-spiro-(9'-fluorene)-1, 2, 4-triazin-5-(4H)-ones as potential anti HIV and anticancer drugs. Pharmazie 1994, 49, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.B.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S. Synthesis of 2-[3, 5- Substituted Pyrazol-1-yl]-4, 6-trisubstituted triazine derivatives as antimalarial agents. Bioorg. Med. Chem. Lett. 2005, 15, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; Srinivas, U.; Jayathirtha, R.; Bhanuprakash, K.; Harakishore, K.; Murthy, U.S.N. Synthesis and antibacterial activity of 2,4,6-trisubstituted s-triazines. Bioorg. Med. Chem. Lett. 2005, 15, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Jha, A.K.; Thakur, A.S.; Dewangan, D. Synthesis and antibacterial activity of some 1, 3, 4-oxadiazole derivatives and their thione analogues. Int. J. Res. Pharma. Bio. Sci. 2011, 2, 215–219. [Google Scholar]
- Saravanan, J.; Mohan, S.; Roy, J.J. Synthesis of some 3-substituted amino-4,5-tetramethylene thieno[2,3-d][ 1,2,3]-triazin-4(3H)-ones as potential antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 4365–4369. [Google Scholar] [CrossRef] [PubMed]
- Sangshetti, J.N.; Shinde, D.B. One pot synthesis and SAR of some novel 3-substituted 5,6-diphenyl-1,2,4-triazines as antifungal agents. Bioorg. Med. Chem. Lett. 2010, 20, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.C.A.; Briggs, E.; Clarke, E.D.; Whittingham, W.G. Synthesis and SAR studies of novel antifungal 1,2,3-triazines. Bioorg. Med. Chem. Lett. 2007, 17, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Dawane, B.S.; Kadam, S.N.; Shaikh, B.M. An efficient synthesis of 1, 2, 4-triazine derivatives and their in vitro antimicrobial activity. Der. Pharmacia Lett. 2010, 2, 126–131. [Google Scholar]
- Yang, R.; Kaplan, P.A. Reaction of isothiourea with 2,3-diaza-3-pentenedioic anhydride: A solid-phase synthesis of 3-amino-1,2,4-triazin-5(4H)-ones. Tetrahedron Lett. 2001, 42, 4433–4435. [Google Scholar] [CrossRef]
- Oettmeier, W.; Hilp, U.; Draber, W. Structure-activity relationships of triazinone herbicides on resistant weeds and resistant chlamydomonas reinhardtii. Pestic. Soc. 1991, 33, 399–409. [Google Scholar] [CrossRef]
- Kranz, E.; Santel, H.; Luerssen, K. New 6-cyclo-butyl-1, 2, 4-triazinone derivatives—useful as herbicides and plant growth regulators. DE: 3917043 A1, 1990. [Google Scholar]
- Kaushik, D.; Verma, T.; Madaan, K. 2-(Benzoylamino)-3-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)acrylic acid. Molbank 2011, 2011, M726. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).