Abstract
The title compound, (2E)-3-(4-dimethylaminophenyl)-1-(2,5-dimethylfuran-3-yl)-prop-2-en-1-one (3) was synthesized in high yield by reaction of 3-acetyl-2,5-dimethylfuran and 4-dimethylaminobenzaldehyde in the presence of 30% NaOH solution. The compound was fully characterized from its IR, 1H NMR, 13C NMR, GC-MS data and elemental analysis.
Chalcones are characterized by the α,β-unsaturated carbonyl system [1], which is important in elucidating the mechanism of transamination and racemisation reactions in biological systems. Chalcones have been studied as antimalarial [2], antifungal [3], anticancer [4], antioxidant [5], tyrosinase inhibitory [6], antiinflammatory [7] and antibacterial agents [8]. Beyond these very important applications in biological chemistry, chalcones have attracted some attention in the field of material sciences including non-linear optics (NLO) [9], optical limiting [10], electrochemical sensing [11] and Langmuir film [12]. They are also used as intermediates for the formation of various hetero-cyclic compounds such as pyrimidines, pyrazolines, pyrazoles, thiazines [13]. These observations led us to synthesize a new chalcone from 3-acetyl-2,5-dimethylfuran and 4-dimethylaminobenzaldehyde.
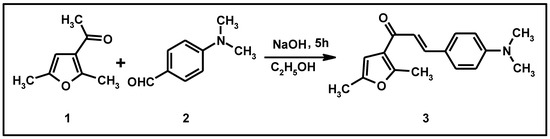
Figure 1.
Synthesis of compound (3).
Figure 1.
Synthesis of compound (3).
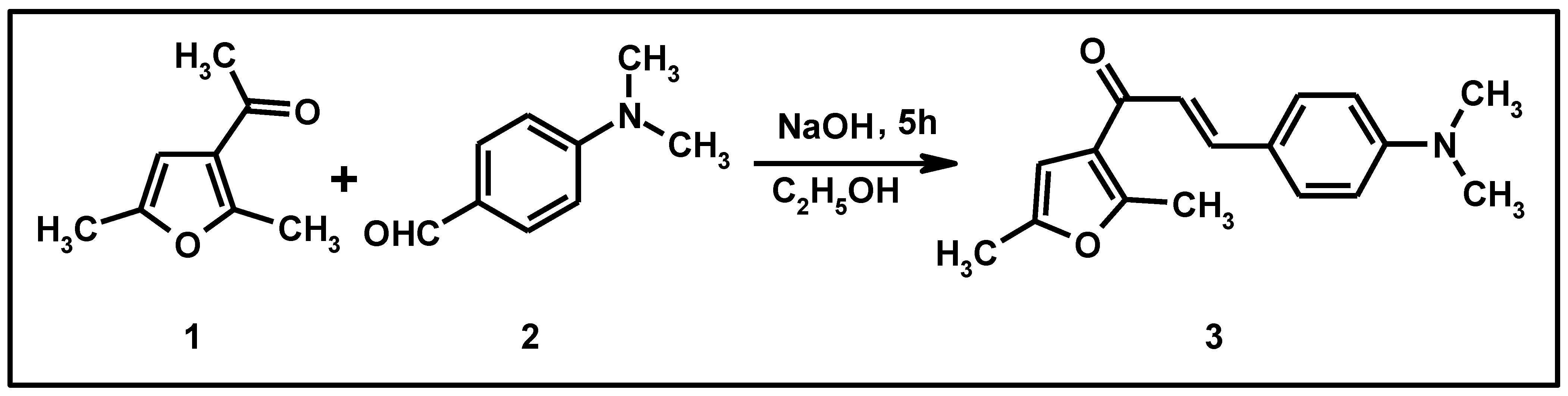
A solution of 3-acetyl-2,5-dimethylfuran (0.46 g, 0.0033 mol) and 4-dimethylaminobenzaldehyde (0.5 g, 0.0033 mol) in an ethanolic solution of NaOH (3.0 g in 10 mL of ethanol) was stirred for 16 h at room temperature. The solution was poured into ice-cold water of pH ~2 (pH adjusted by HCl). The semi-solid separated was collected.
Yellow solid: yield: 86%; semi-solid.
GC-MS m/z (rel. int.%): 270 (58) [M+1]+.
IR (KBr) vmax cm−1: 3061 (Ar-H), 2903 (C-H), 1640 (C=O), 1559 (C=C).
1H NMR (Bruker, 600 MHz, CDCl3): δ (ppm) 7.63 (d, 2H, J = 8.0 Hz), 6.72 (d, 2H, J = 8.0 Hz), 7.55 (d, 1H, C=CH, J = 15.4 Hz), 7.21 (d, 1H, CH=C, J = 15.4 Hz), 6.74 (s, 1H, Ar-H), 3.17 (s, NCH3), 3.04 (s, NCH3), 2.26 (s, CH3), 2.08 (s, CH3).
13C NMR (150 MHz, CDCl3): δ (ppm) 184, 156, 152, 150, 142, 130, 124, 122, 123, 119, 112, 113, 105, 40, 39, 14, 13.
Anal. calcd. for C17H19NO2: C, 75.81, H, 7.11, N, 5.20. Found: C, 75.76, H, 7.09, N, 7.16.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the Chemistry Department and the Center of Excellence for Advanced Materials Research, King Abdul Aziz University, Jeddah, Saudi Arabia for providing the research facilities.
References
- Asiri, A.M.; Khan, A.S. 2E,2'E-3,3'-(1,4-Phenylene)bis(1-(2,5-dimethylfuran-3-yl)prop-2-en-1-one. Molbank 2010, 2010, M694. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.M.; Bhasin, V.K. Antimalarial pharmacodynamics of chalcone derivatives in combination with artemisinin against Plasmodium falciparum in vitro. Eur. J. Med. Chem. 2009, 44, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Dominguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortes, J.C.G.; Ribas, J.C.; Devia, C.; Rodriguez, A.M.; Ricardo, D.; Enriz, R.D. In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Ducki, S. The development of chalcones as promising anticancer agents. IDrugs 2007, 1, 42–46. [Google Scholar]
- Yaylı, N.; Ucuncu, O.; Aydın, E.; Gok, Y.; Yaşar, A.; Baltacı, C.; Yıldırım, N.; Kucuk, M. Stereoselective photochemistry of heteroaryl chalcones in solution and the antioxidant activities. J. Photochem. Photobiol. 2005, 169, 229–234. [Google Scholar] [CrossRef]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Hung, C.-F.; Yang, S.-C.; Wang, J.-P.; Won, S.-J.; Lin, C.-N. Synthesis and cytotoxic, anti-inflammatory, and anti-oxidant activities of 2′,5′-dialkoxylchalcones as cancer chemopreventive agents. Bioorg. Med. Chem. 2008, 16, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Avila, H.P.; Smania, E.F.A.; Monache, F.D.; Junior, A.S. Structure–activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Veerendra, B.; Shivananda, M.K. Non-linear optical properties of new arylfuranylpropenones. J. Crystal Growth 2004, 263, 532–535. [Google Scholar] [CrossRef]
- Poornesh, P.; Shettigar, S.; Umesh, G.; Manjunatha, K.B.; Kamath, K.P.; Sarojini, B.K.; Narayana, B. Nonlinear optical studies on 1,3-disubstituent chalcones doped polymer films. Optical Mater. 2009, 31, 854–859. [Google Scholar] [CrossRef]
- Delavaux-Nicot, B.; Maynadie, J.; Lavabre, D.; Fery-Forgues, S. Ca2+ vs. Ba2+ electrochemical detection by two disubstituted ferrocenyl chalcone chemosensors. Study of the ligand–metal interactions in CH3CN. J. Organomet. Chem. 2007, 692, 874–886. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, F.; Lei, X.; Yang, L.; Xu, S.; Duan, X. In situ growth of layered double hydroxide films on anodic aluminum oxide/aluminum and its catalytic feature in aldol condensation of acetone. Chem. Eng. Sci. 2008, 63, 4055–4062. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A. Synthesis and Anti-bacterial Activities of a Bis-chalcone Derived from Thiophene and Its Bis-cyclized Products. Molecules 2011, 63, 523–531. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
