Abstract
The title compound, (2E)-1-(2,5-dimethyl-3-thienyl)3-(4-nitrophenyl)propenone (3) was synthesized in high yield by reaction of 3-acetyl-2,5-dimethythiophene and 4-nitrobenzaldehyde in the presence of sodium hydroxide. The structure of the compound was fully characterized by IR, 1H NMR, 13C NMR, GC-MS spectral analysis and elemental analysis.
1,3-Diaryl-2-propen-1-ones (chalcones) are products of condensation of simple or substituted aromatic aldehydes with simple or substituted acetophenones in the presence of alkali [1]. Chalcones constitute an important group of natural products and some of them possess a wide range of biological activities such as antimicrobial, anticancer, antitubercular, antiviral, etc. Recent studies on biological evaluation of chalcones revealed their potential to be antimalarial [2], antifungal [3], anticancer [4], antioxidant [5], tyrosinase inhibitory [6], anti-inflammatory [7] and antibacterial [8]. Some derivatives of chalcones are used as sweeteners, drugs, and sunscreen agents [9]. They are also well-known intermediates for the synthesis of various heterocyclic compounds such as pyrimidines, pyrazolines, pyrazoles, or thiazines [10]. These observations led us to synthesize a new chalcone from 3-acetyl-2,5-dimethythiophene and 4-nitrobenzaldehyde in analogy to a previous report [11].
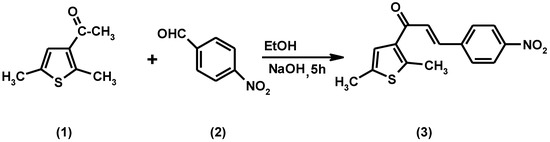
Scheme 1.
Synthesis of the title compound (3).
A solution of 3-acetyl-2,5-dimethythiophene (0.38 g, 0.0025 mol) and 4-nitrobenzaldehyde (0.37 g, 0.0025 mol) in an ethanolic solution of NaOH (3.0 g in 10 mL of ethanol) was stirred for 16 h at room temperature. The solution was poured into ice-cold water of pH ~2 (pH adjusted by HCl). The solid which separated was filtered and crystallized from methanol/chloroform.
Light-yellow solid: yield: 78%; m.p. 130–131 °C.
GC-MS m/z (rel. int. %): 289 (62) [M+1]+.
IR (KBr) vmax cm−1: 3012 (Ar-H), 2926 (C-H), 1628 (C=O), 1568 (C=C).
1H NMR (600 MHz, DMSO-d6) (δ/ppm): 8.47 (d, J = 1.8 Hz), 8.23 (d, J = 1.2 Hz), 7.73 (d, C=CH, J = 15.6 Hz), 7.40 (d, CH=C, J = 15.6 Hz), 7.89 (d, J = 7.2 Hz), 7.61 (d, J = 7.8 Hz), 7.27 (s, Ar-H), 2.72 (s, CH3), 2.39 (s, CH3).
13CNMR (150 MHz, CDCl3) δ: 185.23, 148.66, 148.62, 140.32, 136.79, 136.01, 135.68, 134.31, 129.98, 127.45, 125.76, 124.43, 122.16, 16.06, 15.05.
Anal. calc. for C15H13NO3S: C, 62.70, H, 4.56, N, 4.87. Found: C, 62.66, H, 4.52, N, 4.83.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the deanship of scientific research for the financial support of this work via Grant No. (3-045/430).
References
- Asiri, A.M.; Khan, A.S. 1-(2,5-Dimethyl-3-thienyl)-3-(2,4,5-trimethyphenyl)prop-2-en-1-one. Molbank 2010, 2010, M692. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Antimalarial pharmacodynamics of chalcone derivatives in combination with artemisinin against Plasmodium falciparum in vitro. Eur. J. Med. Chem. 2009, 44, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Dominguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortes, C.G.; Ribas, J.C.; Devia, C.; Rodriguez, A.M.; Enriz, R.D. In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Kamal, A.; Ramakrishna, G.; Raju, P.; Viswanath, A.; Ramaiah, M.J.; Balakishan, G.; Pal-Bhadra, M. Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorg. Med. Chem. Lett. 2010, 20, 4865–4869. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.N.; Wang, L.P.; Tao, R.X. Expression patterns of defence genes and antioxidant defence responses in a rice variety that is resistant to leaf blast but susceptible to neck blast. Physiol. Mol. Plant P. 2009, 74, 167–174. [Google Scholar] [CrossRef]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2,4-substituted resorcinol moiety. Bioorg. Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tuchinda, P.; Reutrakul, V.; Claeson, P.; Pongprayoon, U.; Sematong, T.; Santisuk, T.; Walter, C.; Taylor, W.C. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 2002, 59, 169–173. [Google Scholar] [CrossRef]
- Selvakumar, N.; Kumar, G.S.; Azhagan, A.M.; Rajulu, G.G.; Sharma, S.; Kumar, M.K.; Das, J.; Iqbal, J.; Trehan, S. Synthesis, SAR and antibacterial studies on novel chalcone oxazolidinone hybrids. Eur. J. Med. Chem. 2007, 42, 538–583. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Steingrover, K.; Rao, M.S. Isolation and synthesis of chalcones with different degrees of saturation. Phytochemisy 2002, 61, 931–936. [Google Scholar] [CrossRef]
- Sharma, M.; Chaturvedi, V.; Manju, Y.K.; Bhatnagar, S.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S. Substituted quinolinyl chalcones and quinolinyl pyrimidines as a new class of anti-infective agents. Eur. J. Med. Chem. 2009, 44, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Batterjee, S.; Taib, L.A. Stereoselective crossed-aldol condensation of 3-acetyl-2,5-dimethylthiophene/furan with aromatic aldehydes in water: Synthesis of (2E)-3-aryl-1-(thien-3-yl/fur-3-yl)-prop-2-en-1-ones. Ind. J. Chem. B 2006, 45B, 1936–1941. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).