Abstract
(Benzoylamino)methyl 4-acetyloxybenzoate (3) was obtained in a reaction of benzamidomethylation of 4-acetyloxybenzoic acid (2) with (benzamidomethyl)triethyl-ammonium chloride (1).
In the course of our work on the synthesis of some new derivatives of 4-hydroxybenzoic acid [1,2,3], an additional compound, (benzoylamino)methyl 4-acetyloxybenzoate (3) was synthesized.
4-Acetyloxybenzoic acid (2) was benzamidomethylated with (benzamidomethyl)triethylammonium chloride (1) as a reagent for benzamidomethylation [4]. The reaction was performed in dioxane suspension of 1 in the presence of a small amount of triethylamine at 50 °C (Scheme 1). At the end of the reaction, water was added to the reaction mixture to precipitate the product. However, the crystals of 3 were formed slowly, which is unusual comparing to similar procedures for isolation of benzamidomethyl esters [4]. The maximal yield of almost pure crude product was 48%.
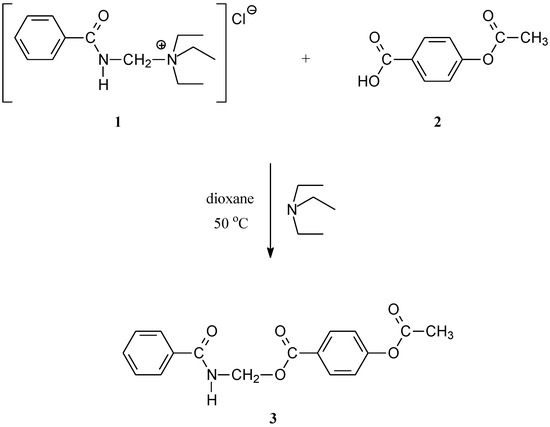
Scheme 1.
Synthetic routes to the title compound 3.
Experimental
Compound 2 is not commercially available and it was synthesised as described previously [5].
(Benzoylamino)methyl 4-acetyloxybenzoate (3)
To a suspension of 1 (0.674 g, 2.49 mmol) in dioxane (30 mL), 2 (0.370 g, 2.05 mmol) and TEA (0.1 mL, 0.72 mmol) were added. The mixture was stirred and heated at 50 °C for 20 h. After cooling, cold water was added to the mixture until a white precipitate occurred. Colorless crystals were collected by simple filtration. Purification was performed by dissolving the crystals in dioxane and by precipitation with water. Significant loss of 3 during the purification was observed.
Melting point of pure crystals: 112–114 °C.
FT-IR (KBr): 3,343 cm−1 (νNH); 1,759 and 1,726 cm−1 (νOC=O); 1,657 cm−1 Amide I; 1,534 cm−1 Amide II.
1H-NMR (250 MHz, DMSO-d6): δ/ppm 9.69 (t, J = 6.7 Hz, 1H, NH); 8.01–7.27 (m, 9H, Ar); 5.60 (d, J = 6.7 Hz, 2H, N-CH2-O); 2.29 (s, 3H, CH3)
13C-NMR (63 MHz, DMSO-d6): δ/ppm 168.9 (C=O); 167.2 (C=O); 164.8 (C=O); 65.9 (CH2); 20.9 (CH3); Ar: 154.5, 133.2, 132.1, 130.9, 128.6, 127.6, 127.1 and 122.4.
Anal. Calcd. (found) for C17H15NO5: C, 65.17 (64.99); H, 4.82 (5.03); N, 4.47 (4.62).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Popovski, E.; Mladenovska, K. (Benzoylamino)methyl 4-hydroxybenzoate. Molbank 2010, 2010, M658. [Google Scholar] [CrossRef]
- Popovski, E.; Mladenovska, K.; Panovska, A.P. (Benzoylamino)methyl 4-[(Benzoylamino)methoxy]benzoate. Molbank 2011, 2011, M711. [Google Scholar] [CrossRef]
- Popovski, E.; Mladenovska, K. Panovska, A.P. Methyl 4-[(Benzoylamino)methoxy]benzoate. Molbank 2011, 2011, M712. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acids. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple method for benzamidomethylation of phenols in water solution. Synth. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).