Abstract
A new compound, ethyl 3,5-dimethyl-4-[(4-phenyl-1,3-thiazol-2-yl)carbamoyl]-1H-pyrrole-2-carboxylate (3) was synthesized by the amination method. The synthesized compound (3) was characterized by IR, 1H-NMR, 13C-NMR, mass spectral data and elemental analysis.
1. Introduction
Thiazoles are an important class of natural and synthetic compounds. Thiazole derivatives display a wide range of biological activities such as anesthetic [1] and anti-inflammatory [2]. In view of their pharmaceutical applications, the synthesis of thiazoles is important. Here, the preparation and characterization of a new thiazole derivative is reported.
2. Results and Discussion
The two educts, diethyl 3,5-dimethyl-1H-pyrrole-2,4-dicarboxylate (1) and 4-phenyl-1,3-thiazol-2-amine (2) were synthesized according to previously reported methods [3,4,5,6]. The title compound, ethyl 3,5-dimethyl-4-[(4-phenyl-1,3-thiazol-2-yl)carbamoyl]-1H-pyrrole-2-carboxylate (3) was synthesized by an amination reaction [7,8]. The 1H-NMR spectrum of compound (1) showed a quartet at δ 4.20 and a triplet at δ 1.30, corresponding to -COOCH2CH3 and -COOCH2CH3 protons in the 2- and 4-position of the pyrrole ring. The 1H-NMR spectrum (Figure 2) of compound (3) showed a singlet at δ 8.11, corresponding to the -CONH proton in the 4-position of the pyrrole ring. A doublet observed at δ 3.87 and δ 2.18 was attributed to the CH3 protons at 3- and 5-position in the pyrrole ring. A 1D NOE spectrum (Figure 3a and Figure 3b) of compound (3) showed interactions between the amide-NH proton and the CH3 protons at the 3- and 5-position, respectively, thus confirming that the -CONH group is located in 4-position of the pyrrole ring. No signal enhancement was obtained on irradiation of the NH proton at the 1-position of the pyrrole ring. The 13C-NMR spectrum (Figure 4) of compound (3) showed signals at δ 156.9 and δ 166.0, corresponding to the –COOEt group at the 2-position and the –CONH group at the 4-position of the pyrrole ring. The mass spectra (EI) of compound (3) showed the molecular ion peak at m/z 370.43 (M+ + 1, 12%), consistent with the assigned molecular formula C19H19N3O3S.
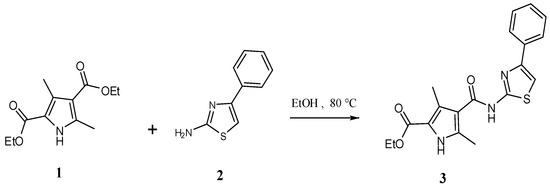
Scheme 1.
Synthesis of the title compound (3).
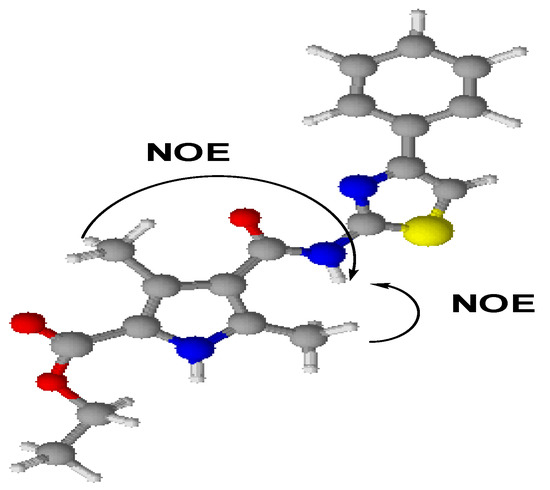
Scheme 2.
Proposed conformation of compound (3).
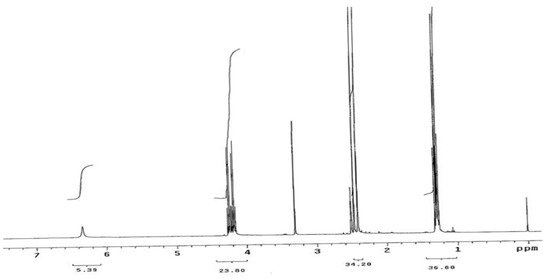
Figure 1.
1H-NMR spectrum of compound (1) (400 MHz, DMSO-d6).
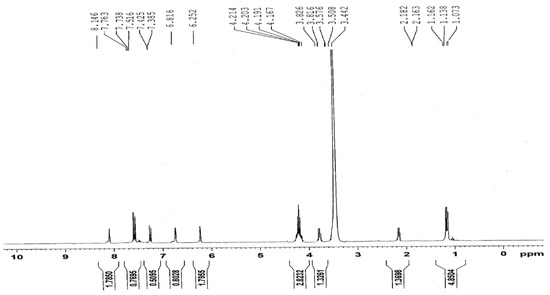
Figure 2.
1H-NMR spectrum of compound (3) (400 MHz, DMSO-d6).
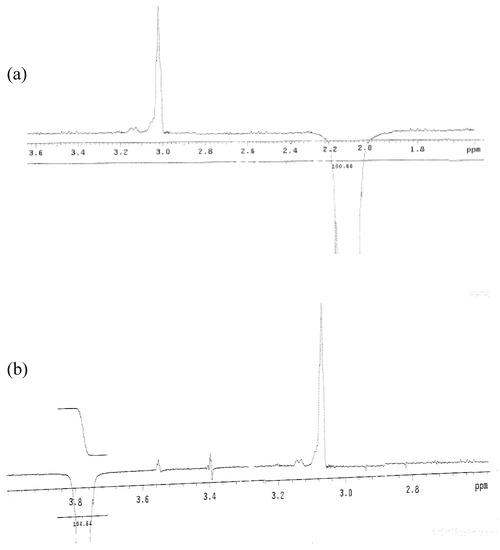
Figure 3.
(a) 1D NOE NMR for CH3 at 5-position, with irradiation of neighboring CONH proton in compound (3) recorded at 400 MHz in DMSO-d6. (b) 1D NOE NMR for CH3 at 2-position, irradiated with neighboring CONH proton in compound (3) recorded at 400 MHz, in DMSO-d6.
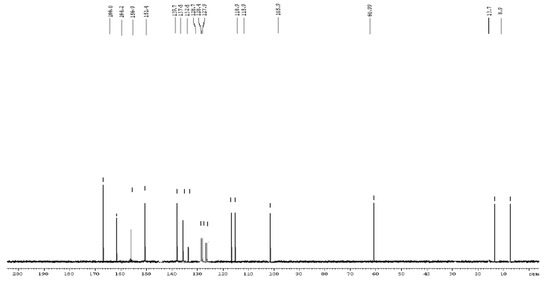
Figure 4.
13C-NMR spectrum of compound (3) (100 MHz, DMSO-d6).
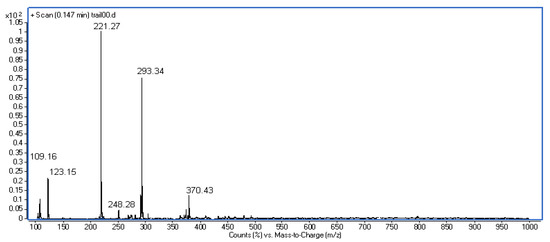
Figure 5.
Mass spectrum (EI) of compound (3).
3. Experimental
The melting point was determined in an open capillary tube and it is uncorrected. The IR spectrum was recorded for a KBr pellet on a Shimadzu 8201pc (4000–400 cm-1). The 1H-NMR and 13C-NMR spectra were recorded on a Bruker DRX-400 and a Varian Mercury Plus 400 at 400 MHz (1H) and 100 MHz (13C), respectively. The mass spectrum (EI) was recorded on a Jeol JMS D-300 spectrometer operating at 70 eV. Elemental analysis (C, H, N and S) were carried out using a Varian Elemental Analyzer EL III. The purity of the compound was checked by thin layer chromatography (TLC) with silica gel plates.
3.1. Synthesis of ethyl 3,5-dimethyl-4-[(4-phenyl-1,3-thiazol-2-yl)carbamoyl]-1H-pyrrole-2-carboxylate (3)
A mixture of diethyl 3,5-dimethyl-1H-pyrrole-2,4-dicarboxylate (1) (2.39 g, 0.01 mol), 4-phenyl-1,3-thiazol-2-amine (2) (1.78 g, 0.01 mol) and absolute ethanol (30 mL) was heated under reflux for 48 h. The reaction mixture was cooled and poured into crushed ice. The obtained solid was filtered off and washed with water. The filtered solid was purified by recrystallisation from absolute ethanol to give the title compound (3) as a yellow solid (1.20 g, 28%).
Melting point: 147 °C
IR (KBr, cm–1): υ 3350 (N-Hstr), 3050 (C-Hstr of phenyl ring), 2953 (C-Hstr of CH3), 1755 (C=O, ester), 1685 (-HN-C=O), 1626 (C=N), 712 (C-S-C).
1H-NMR (DMSO-d6, 400 MHz): δ 8.11 (s, 1H, NH-CO), 7.32–7.74 (m, 5H, Ph), 6.25 (s, 1H, pyrrole NH), 6.85 (s, 1H, thiazole-H), 4.20 (q, 2H, J = 18.8 Hz, OCH2CH3), 3.87 (d, 3H, J = 7.6 Hz, 3-CH3), 2.18 (d, 3H, J = 4 Hz, 5-CH3), 1.30 (t, 3H, J = 16.4 Hz, OCH2CH3).
13C-NMR (DMSO-d6, 100 MHz): δ 166.0 (NHCO), 164.2 (thiazole 2-C), 156.9 (C-COOEt at 2-position in pyrrole ring), 151.4 (thiazole 4-C), 137.5 (pyrrole 3-C), 139.7 (pyrrole 5-C), 127.9, 128.4, 128.7, 132.1 (phenyl-C), 118.8 (pyrrole 2-C), 115.7 (pyrrole 4-C), 105.0 (thiazole 5-C), 60.2 (OCH2CH3 ), 13.8 (5-CH3), 8.9 (3-CH3).
MS (EI): m/z = 370.43 (M+ + 1, 12%), 293.34 (75%), 248.28 (5%), 221.27 (100%), 123.15 (22%), 109.16 (10%).
Elemental analysis: Calcd. for C19H19N3O3S (MW = 369.45): C, 61.76%; H, 5.18%; N, 11.37%; S, 8.67%. Found: C, 61.71%; H, 5.14%; N, 11.33%; S, 8.64%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
We wish to thank the state Government of Tamil Nadu, India, for providing a state government fellowship for financial support. We sincerely thank M. Sheik Mohamed, Principal, Jamal Mohamed College, for providing laboratory facilities.
References and Notes
- Geronikaki, A.; Theophilidis, G. Synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. Eur. J. Med. Chem. 1992, 27, 709–716. [Google Scholar] [CrossRef]
- Giridhar, T.; Reddy, R.B.; Prasanna, B.; Chandra Mouli, G.V.P. Amino thiozoles: Part I- Synthesis and pharmacological evalution. Indian J. Chem. 2001, 40B, 1279–1281. [Google Scholar]
- Fischer, H. 2,4-Dimethyl-3,5-dicarbethoxypyrrole. Org. Synth. 1935, 15, 17. [Google Scholar]
- Fabiano, E.; Golding, B.T. On the mechanism of pyrrole formation in the Knorr pyrrole synthesis and by porphobilinogen synthesis. J. Chem. Soc. Perkin Trans.1 1991, 12, 3371–3375. [Google Scholar] [CrossRef]
- King, L.C.; Hlavacek, R.J. Reaction of ketones with iodine and thiourea. J. Am. Chem. Soc. 1950, 72, 3722–3725. [Google Scholar] [CrossRef]
- Siddiqui, H.L.; Iqbal, A.; Ahmad, S.; Weaver, G.W. Synthesis and spectroscopic studies of new Schiff bases. Molecules 2006, 11, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.A.; Bondock, S.; Rabie, R.; Etman, H.A. Cyanoacetamide derivatives as synthons in heterocyclic synthesis. Turk. J. Chem. 2008, 32, 259–286. [Google Scholar]
- Refaat, H.M.; Moneer, A.A.; Khalil, O.M. Synthesis and antimicrobial activity of certain novel quinoxalines. Arch. Pham. Res. 2004, 27, 1093–1098. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).