Abstract
The title compound 1 was synthesized by the coupling reaction of 6,7,8,10-tetra-O-benzyl-1,2,3,4-tetradeoxy-α-d-gluco-dec-5-ulopyranose (2) with 2,3,4,6-tetra-O-benzyl-d-glucopyranose (3) in the presence of 5 mol% bismuth(III) triflate in dichloromethane at 0 °C.
The addition reaction of RLi (or RMgX) to a sugarlactone derivative is an established technique for synthesizing some artificial ketoses whose anomeric carbons are bound to functional groups, such as alkyl, alkynyl, alkenyl, and aryl groups via a carbon–carbon linkage [1]. As the ketoses prepared by this method from naturally occurring aldoses retain the ring structures of the starting aldoses, they are regarded as the analogues of aldose. Currently, these ketoses form a new class of carbohydrate reagents useful for synthesizing some valuable and complicated compounds such as enzyme inhibitors [2,3,4,5], oligosaccharide mimics [6,7], spiroketals [8,9,10,11,12,13], exo-glycals [14,15], and C- or O-glycosides [16,17,18].
Our former research revealed the synthesis of non-reducing disaccharides by the coupling reaction of benzylated 1-deoxy-α-d-gluco- or d-manno-hept-2-ulopyranoses with 1-hydroxy-aldopyranose derivatives using 5 mol% of bismuth(III) triflate (Bi(OTf)3) or bis(trifluoromethane)sulfonamide as an activator [19,20]. The synthesized non-reducing disaccharides are the mimics of trehalose which is composed of two glucose molecules linked to each other by an α-glucopyranosidic group. As trehalose is well-known for its various biological functions such as the suppressive effect on osteoporosis progress [21], it is important to synthesize various kinds of trehalose mimics which are expected to show novel useful functions. This paper describes the synthesis of the title compound 1 by the coupling reaction of 6,7,8,10-tetra-O-benzyl-1,2,3,4-tetradeoxy-α-d-gluco-dec-5-ulopyranose (2) [22] with 2,3,4,6-tetra-O-benzyl-d-glucopyranose (3).
Compound 1 was obtained in a good yield of 70% by the coupling reaction of 2 with 3 in the presence of 5 mol% Bi(OTf)3 in dichloromethane at 0 °C for 3 h. Various NOE and 1H NMR experiments were performed with 1. The NOE interaction between H-6’ and H-4’ was observed. This observation inevitably indicates the equatorial orientation of the butyl group and determines the α-ketopyranosidic linkage. The α-aldopyranosidic linkage is confirmed by the coupling constant (J = 3.5 Hz) of H-1 [23]. Thus, both of the glycosidic linkages of 1 are α [24]. Based on TLC monitoring of the reaction, the self-condensation product from 2 or 3 was not observed at all.
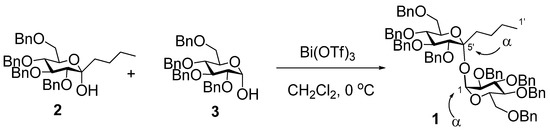
Scheme 1.
Coupling reaction of 2 with 3 in the presence of Bi(OTf)3 to produce 1.
Experimental
6,7,8,10-Tetra-O-benzyl-1,2,3,4-tetradeoxy-α-d-gluco-dec-5-ulopyranosyl 2,3,4,6-tetra-O-benzyl-α-d-glucopyranoside (1)
To a solution of Bi(OTf)3 (4.8 mg, 0.007 mmol), 2 (52.9 mg, 0.10 mmol) and CaSO4 (ca. 100 mg) in CH2Cl2 (3.5 mL) was added 3 (87.2 mg, 0.15 mmol) at 0 oC under an Ar atmosphere. The resulting mixture was stirred for 3 h. The reaction was then quenched by addition of a sat. NaHCO3 solution (5 mL). The reaction mixture was extracted with CH2Cl2, and the organic layer was washed with water and a sat. NaCl solution. After the organic layer was dried over Na2SO4, the solvent was evaporated under reduced pressure. The crude product was purified by preparative silica gel TLC (ethyl acetate/hexane = 1/3, Rf = 0.48) to give 1 (77.1 mg, 70%) as a colorless oil. [α]D26 + 25° (c 3.9, CHCl3). 1H NMR (600 MHz, CDCl3): δ 0.83 (3H, t, J = 6.9 Hz, H-1’), 1.08–1.17 (2H, m, Ha-2’, Ha-3’), 1.24–1.31 (1H, m, Hb-2’), 1.40–1.45 (1H, m, Hb-3’), 1.88–1.90 (2H, m, H-4’), 3.37 (1H, d, J = 11.0 Hz, Ha-10’), 3.42–3.45 (2H, m, Hb-10’, Ha-6), 3.54 (1H, d, J = 9.0 Hz, H-6’), 3.56 (1H, dd, J = 3.4 Hz, J = 9.6 Hz, H-2), 3.57–3.59 (1H, m, Hb-6), 3.59 (1H, t, J = 10.3 Hz, H-8’), 3.67 (1H, t, J = 9.6 Hz, H-4), 4.08 (1H, t, J = 9.6 Hz, H-3), 4.10 (1H, t, J = 9.7 Hz, H-7’), 4.22–4.23 (1H, m, H-5), 4.30–4.32 (1H, m, H-9’), 4.38 (1H, d, J = 13.1 Hz, CH2Ph), 4.40 (1H, d, J = 13.7 Hz, CH2Ph), 4.52 (1H, d, J = 12.4 Hz, CH2Ph), 4.54 (1H, d, J = 12.4 Hz, CH2Ph), 4.56–4.61 (3H, m, CH2Ph), 4.66 (1H, d, J = 11.7 Hz, CH2Ph), 4.69 (1H, d, J = 12.4 Hz, CH2Ph), 4.80-4.95 (7H, m, CH2Ph), 5.38 (1H, d, J = 3.5 Hz, H-1), 7.12–7.31 (40H, m, Ph). 13C NMR (150 MHz, CDCl3): δ 14.1 (C-1’), 23.0 (C-2’), 26.6 (C-3’), 33.8 (C-4’), 68.4 (C-6), 68.7 (C-10’), 70.5 (C-5), 71.3 (C-9’), 73.0 (CH2Ph), 73.1 (CH2Ph), 73.3 (CH2Ph), 74.56 (CH2Ph), 74.65 (CH2Ph), 74.66 (CH2Ph), 75.3 (CH2Ph), 75.4 (CH2Ph), 78.2 (C-4), 78.6 (C-8’), 80.2 (C-2), 80.4 (C-6’), 81.3 (C-3), 83.0 (C-7’), 89.5 (C-1), 103.0 (C-5’), 127.2–128.3 (Ph), 138.0–138.9 (Ph). HRMS (ESI): m/z calcd for C72H78O11Na+: 1141.5436; found: 1141.5456. Anal. Calcd for C72H78O11•1.5 H2O: C, 75.43; H, 7.12. Found: C, 75.31; H, 7.13.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Yamanoi, T.; Matsuda, S. Reactions and uses of artificial ketoses. Heterocycles 2009, 79, 163–194 and references cited herein. [Google Scholar] [CrossRef]
- Benltifa, M.; Vidal, S.; Gueyrard, D.; Goekjian, P.G.; Msaddek, M.; Praly, J.P. 1,3-Dipolar cycloaddition reactions on carbohydrate-based templates: Synthesis of spiro-isoxazolines and 1,2,4-oxadiazoles as glycogen phosphorylase inhibitors. Tetrahedron Lett. 2006, 47, 6143–6147. [Google Scholar] [CrossRef]
- Pan, D.; Liu, J.; Senese, C.; Hopfinger, A.J.; Tseng, Y. Characterization of a ligand−receptor binding event using receptor-dependent four-dimensional quantitative structure−activity relationship analysis. J. Med. Chem. 2004, 47, 3075–3088. [Google Scholar] [CrossRef] [PubMed]
- Stolz, F.; Reiner, M.; Blume, A.; Reutter, W.; Schmidt, R.R. Novel UDP-glycal derivatives as transition state analogue inhibitors of UDP-GlcNAc 2-epimerase. J. Org. Chem. 2004, 69, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Somsák, L.; Kovács, L.; Tóth, M.; Ősz, E.; Szilágyi, L.; Györgydeák, Z.; Dinya, Z.; Docsa, T.; Tóth, B.; Gergely, P. Synthesis of and a comparative study on the inhibition of muscle and liver glycogen phosphorylases by epimeric pairs of d-gluco- and d-xylopyranosylidene-spiro-(thio)hydantoins and N-(d-glucopyranosyl) amides. J. Med. Chem. 2001, 44, 2843–2848. [Google Scholar] [CrossRef]
- Namme, R.; Mitsugi, T.; Takahashi, H.; Ikegami, S. Synthesis of PI-88 analogue using novel O-glycosidation of exo-methylenesugars. Tetrahedron Lett. 2005, 46, 3033–3036. [Google Scholar] [CrossRef]
- Li, X.; Ohtake, H.; Takahashi, H.; Ikegami, S. Direct methylenation of partially benzyl-protected sugar lactones by dimethyltitanocene. Synlett 2001, 1885–1888. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamashita, K.; Hotta, T.; Hashimoto, T.; Kikuchi, M.; Nishiyama, H. Synthesis of spirocyclic C-arylglycosides and -ribosides by ruthenium-catalyzed cycloaddition. Chem. Asian J. 2007, 2, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- van Hooft, P.A.V.; Oualid, F.E.; Overkleeft, H.S.; van der Marel, G.A.; van Boom, J.H.; Leeuwenburgh, M.A. Synthesis and elaboration of functionalised carbohydrate-derived spiroketals. Org. Biomol. Chem. 2004, 2, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Takahashi, H.; Ohtake, H.; Shiro, M.; Ikegami, S. Stereoselective synthesis and structure elucidation of spiro-ketodisaccharides. Tetrahedoron 2001, 57, 8053–8066. [Google Scholar] [CrossRef]
- Czernecki, S.; Perlat, M.C. C-Glycosides. 9. Stereospecific synthesis of C-glycosidic spiroketal of the papulacandins. J. Org. Chem. 1991, 56, 6289–6292. [Google Scholar] [CrossRef]
- Hanessian, S.; Ugolini, A. Synthesis of a chiral 1,7-dioxaspiro[5,5]undecene. A model for the spiroacetal subunit of avermectin B1a. Carbohydr. Res. 1984, 130, 261–269. [Google Scholar] [CrossRef]
- Yamanoi, T.; Oda, Y.; Muraishi, H.; Matsuda, S. Synthesis of a novel d-glucose-conjugated 15-crown-5 ether with a spiro ketal structure. Molecules 2008, 13, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, T.; Nara, Y.; Matsuda, S.; Oda, Y.; Yoshida, A.; Katsuraya, K.; Watanabe, M. Synthetic approach to exo-glycals from 1-C-vinyl-d-glycopyranose derivatives via an SN1’-substitution mechanism. Synlett 2007, 785–789. [Google Scholar] [CrossRef]
- Tomooka, K.; Nakamura, Y.; Nakai, T. [2,3]-Wittig Rearrangement using Glucose as a Chiral Auxiliary: Asymmetric Transmission from the Anomeric Center. Synlett 1995, 321–322. [Google Scholar] [CrossRef]
- Matsuda, S.; Matsumura, K.; Watanabe, M.; Yamanoi, T. Synthesis of a partially benzylated derivative of the anhydro-d-altro-heptulose found in Coriaria japonica A. Tetrahedron Lett. 2007, 48, 5807–5810. [Google Scholar] [CrossRef]
- Matsuda, S.; Yamanoi, T.; Watanabe, M. Syntheses of a partially benzylated derivative of the anhydro-d-altro-heptulose found in Coriaria japonica A and of its analogs. Tetrahedron 2008, 64, 8082–8088. [Google Scholar] [CrossRef]
- Lay, L.; Meldal, M.; Nicotra, F.; Panza, L.; Russo, G. Stereoselective synthesis of the C-analogue of β-d-glucopyranosyl serine. Chem. Commun. 1997, 1469–1470. [Google Scholar] [CrossRef]
- Yamanoi, T.; Inoue, R.; Matsuda, S.; Katsuraya, K.; Hamasaki, K. Synthesis of trehalose mimics by bismuth(III) triflate or bis(trifluoromethane)sulfonimide-catalyzed 1-C-methyl-d-hexopyranosylation. Tetrahedron-Asymmetry 2006, 17, 2914–2918. [Google Scholar] [CrossRef]
- A similar paper has been reported after our publication of ref. 19: Namme, R.; Mitsugi, T.; Takahashi, H.; Ikegami, S. Development of ketoside-type analogues of trehalose by using-stereoselective O-glycosidation of ketose. Eur. J. Org. Chem. 2007, 3758–3764. [Google Scholar]
- Nishizaki, Y.; Yoshizane, C.; Toshimori, Y.; Arai, N.; Akamatsu, S.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Disaccharide-trehalose inhibits bone resorption in ovariectomized mice. Nutrition Res. 2000, 20, 653–664. [Google Scholar] [CrossRef]
- Oda, Y.; Yamanoi, T. Trimethylsilyl trifluoromethanesulfonate catalyzed nucleophilic substitution to give C- and N-glucopyranosides derived from d-glucopyranose. Synthesis 2007, 3021–3031. [Google Scholar] [CrossRef]
- Xue, J.L.; Cecioni, S.; He, L.; Vidal, S.; Praly, J.P. Variations on the SnCl4 and CF3CO2Ag-promoted glycosidation of sugar acetates: A direct, versatile and apparently simple method with either α or β stereocontrol. Carbohydr. Res. 2009, 344, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- The formation of the α,α-glycosyl linkages corresponded to our former reaction using 3,4,5,7-tetra-O-benzyl-1-deoxy-α-d-gluco-hept-2-ulopyranose and 3. See ref.19. 2 was more promptly activated by a catalytic amount of Bi(OTf)3 than 3. Therefore, we supposed 2 worked as the glycosyl donor and 3 served as the glycosyl acceptor.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).