Abstract
Lariat ethers are interesting recognition motifs in supramolecular chemistry. The synthesis of a luminescent lariat aza-crown ether with a carboxyl group appended by azide-alkyne (Huisgen) cycloaddition is presented.
1. Introduction
Luminescent crown ether amino acids which bind ammonium ions and signal the binding event by changes of their specific emission properties were published recently [1]. These are versatile building blocks for amino acid and peptide receptors [2,3,4]. Introduction of charged groups [5,6,7,8] may enhance the cation binding affinity of crown ethers [9]. The copper(I) catalyzed dipolar cycloaddition (Huisgen cycloaddition) is known to be a robust ligation method for a variety of differently substituted azides and alkynes [10,11,12]. We present the functionalisation of luminescent aza-crown ethers with anionic podand arm by this method. Such a compound is expected to have enhanced ammonium ion binding properties.
2. Synthesis
The lariat ether was prepared by dipolar azide-alkyne (Huisgen) cycloaddition at the side chain of the propargyl-substituted crown ether (1) with 4-azidobutyric acid (2).
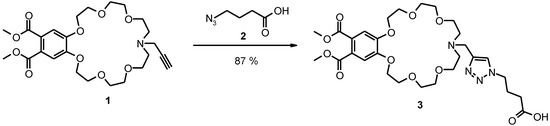
Scheme 1.
Synthesis of the new lariat ether 3 by copper(I) mediated dipolar cycloaddition.
3. Experimental
Crown ether 1 [2] and 4-azidobutyric acid (2) [13] were prepared as published.
14-[4-(4-Methyl-1H-1,2,3-triazol-1-yl)butanoic acid]-6,7,9,10,13,14,15,16,18,19,21,22-dodecahydro-12H-5,8,11,17,20,23-hexaoxa-14-aza-benzocycloheneicosene-2,3-dicarboxylic acid dimethyl ester (3)
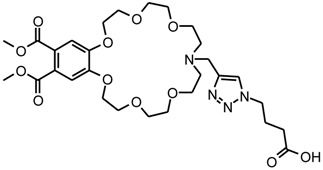
Compound 1 (102 mg, 0.2 mmol) was dissolved together with compound 2 (39 mg, 0.3 mmol) in 1.0 mL of methanol. A solution of copper(II)sulphate pentahydrate (10 mg, 0.02 mmol) in 0.5 mL of water containing sodium ascorbate (16 mg, 0.1 mmol) was added slowly. Under nitrogen atmosphere, the mixture was vigorously stirred for 1 h at room temperature, then for 5 h at 60 °C. It was cooled to room temperature, dichloromethane (9 mL) was added, the aqueous layer was separated and the organic phase was washed with brine (3 mL). After drying over MgSO4, a dry load with a minimum amount of silica gel was prepared. This was transferred to a short column containing a thin layer of silica gel. Ethyl acetate/ethanol 3:1 was used to wash all impurities off the column (Rf (3) = 0.0). The solvent mixture was changed to chloroform/methanol 4:1 to elute compound 3 (Rf (3) = 0.1). The lariat ether (3) appears as colourless glass (110 mg, 0.174 mmol, 87%).
MF: C29H42N4O12–FW: 638.68 g/mol; -IR (neat): ν (cm-1) = 3040 (w), 2949 (m), 2812 (m), 1719 (s), 1598 (m), 1519 (m), 1435 (m), 1349 (m), 1289 (s), 1247 (m), 1199 (m), 1123 (s), 1049 (m), 977 (m), 945 (m), 746 (s), 664 (m); -MS (ESI-MS, CH2Cl2/MeOH + 10 mmol NH4OAc): m/z (%) = 639.2 (100, MH+); -UV (MeOH): λ (ε) = 269 (8600), 225 (30500); -HRMS (PI-LSIMS FAB Glycerine): calc. for C29H43N4O12+: 639.2877, found: 639.2865; -1H-NMR (300 MHz, CDCl3): δ [ppm] = 2.02–2.26 (m, 4 H), 2.85 (m, 2 H), 3.55–3.68 (m, 12 H), 3.69–3.80 (m, 4 H), 3.86 (s, 6 H), 3.83–3.96 (m, 4 H), 4.20 (m, 4 H), 4.35 (t, 2 H, J = 6.3 Hz), 7.18 (s, 2 H), 7.70 (s, 1 H); -13C-NMR (75 MHz, CDCl3): δ [ppm] = 26.1 (-, 1 C), 49.5 (-, 1 C), 49.7 (-, 1 C), 52.6 (+, 2 C), 53.3 (-, 2 C), 53.4 (-, 1 C), 68.4 (-, 2 C), 69.1 (-, 2 C), 69.4 (-, 2 C), 70.3 (-, 2 C), 70.6 (-, 2 C), 114.0 (+, 2 C), 124.1 (+, 1 C), 125.6 (Cquat, 2 C), 143.3 (Cquat, 1 C), 150.5 (Cquat, 2 C), 167.7 (Cquat, 2 C), further signals were not detectable.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (GRK 760) and the University of Regensburg for support of this work.
References and Notes
- Mandl, C.P.; König, B. Luminescent Crown Ether Amino Acids - Selective Binding to N-terminal Lysine in Peptides. J. Org. Chem. 2005, 70, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Späth, A.; König, B. Modular Synthesis of Di- and Tripeptides of Luminescent Crown Ether Aminocarboxylic Acids. Tetrahedron 2009, 65, 690–695. [Google Scholar] [CrossRef]
- Späth, A.; König, B. Ditopic crown ether–guanidinium ion receptors for the molecular recognition of amino acids and small peptides. Tetrahedron 2010, 66, 1859–1873. [Google Scholar] [CrossRef]
- Stadlbauer, S.; Riechers, A.; Späth, A.; König, B. Utilizing Reversible Copper(II) Peptide Coordination in a Sequence-Selective Luminescent Receptor. Chem. Eur. J. 2008, 14, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Bock, H.; Hierholzer, B.; Hollmann., G.; Vögtle, F. Radical Anion-Substituted Crown Ethers as Cages for Metal Cations. Angew. Chem. Int. Ed. 1984, 23, 57–58. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry–Concepts and Perspectives; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Lämsä, M.; Pursiainen, J.; Rissanen, K.; Huuskonen, J. Complexation of Crown Ethers and Podands with Tropylium Cations: Determination of Stability Constants and Crystal Structure of the Dibenzo-24-Crown-8-Tropylium Cation Complex. Acta. Chem. Scand. 1998, 52, 563–570. [Google Scholar] [CrossRef]
- Sharma, C.V.K.; Clearfield, A. “Macrocyclic Leaflets”. J. Am. Chem. Soc. 2000, 122, 1558–1559. [Google Scholar] [CrossRef]
- Gokel, G.W.; Arnold, K.A.; Delgado, M.; Echeverria, M.; Gatto, V.J.; Gustowski, D.A.; Hernandez, J.; Kaifer, A.; Miller, S.R.; Echegoyen, L. Lariat Ethers: from Cation Complexation to Supramolecular Assemblies. Pure Appl. Chem. 1988, 60, 461–465. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Krivopalov, V.P.; Shkurko, O.P. 1,2,3-Triazole and its Derivatives. Development of Methods for the Formation of the Triazole Ring. Russ. Chem. Rev. 2005, 74, 339–379. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The Growing Applications of Click Chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Zhang, K.; Jiang, X. Visual Detection of Copper(II) by Azide- and Alkyne-Functionalized Gold Nanoparticles Using Click Chemistry. Angew. Chem., Int. Ed. 2008, 47, 7454–7456. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).