Abstract
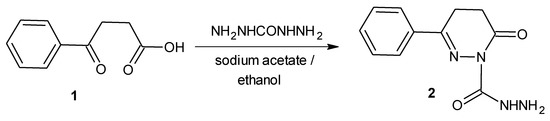
We report herein the synthesis of 6-oxo-3-phenyl-5,6-dihydropyridazine-1(4H)-carbohydrazide from β-benzoylpropionic acid and carbohydrazide by refluxing in absolute ethanol in presence of sodium acetate. The structure of the newly synthesized compound was established on the basis of IR, 1H-NMR, 13C-NMR and mass spectral data.
1. Introduction
Pyridazines and their 3-oxo derivatives (i.e., pyridazinones) are important 1,2-diazine-containing heterocyclic compounds having diverse pharmacological activity such as antihypertensive, antidiabetic, anti-inflammatory, antinociceptive and antimicrobial [1,2,3,4,5,6]. One of the general methods for the synthesis of these compounds involves condensation of 3-aroylpropionic or β-acetylacrylic acid with alkyl or arylhydrazines. Keeping in mind the medicinal utility of pyridazinones, we report herein the synthesis of 6-oxo-3-phenyl-5,6-dihydropyridazine-1(4H)-carbohydrazide.
2. Results and Discussions
In the present study, synthesis of 6-oxo-3-phenyl-5,6-dihydropyridazine-1(4H)-carbohydrazide 2 is reported from β-benzoylpropionic acid using carbohydrazide as a hydrazine derivative by refluxing in absolute ethanol in presence of anhydrous sodium acetate. The structure of compound 2 was established on the basis of IR, 1H-NMR, 13C-NMR and mass spectral data. In the 1H-NMR spectrum of compound 2, the peaks due to the –CONHNH2 group were observed as a singlet at 9.78 ppm (CONH) and another signal of NH2 which overlaps with the multiplet of phenyl protons at 7.27–7.92 ppm. The two CH2 groups of the pyridazinone ring appear as triplet-like signals at 2.35 and 2.87 ppm. Moreover, in the 13C-NMR spectrum the signals due to the CH2CH2 group of the pyridazinone ring appear at 29.4 and 36.1 ppm, while the peaks due to the carbonyl carbon atoms (C=O) are observed at 169.8 and 178.3 ppm. The MS-(ESI) spectrum of compound 2 exhibits an M+1 peak at m/z = 233. The values are in complete agreement with the structure assigned.
3. Experimental
The starting material β-benzoylpropionic acid 1 was synthesized based on a literature method [7].
3.1. 6-Oxo-3-phenyl-5,6-dihydropyridazine-1(4H)-carbohydrazide 2
To a solution of β-benzoylpropionic acid (0.01 mol) in absolute ethanol (30 mL) were added carbohydrazide (0.01 mol) and sodium acetate, and the mixture was refluxed for 6 h. After completion of the reaction, ethanol was distilled off and the residue was poured into cold water. The solid which separated was filtered and washed with water. The product was dried in air and crystallized from ethanol.
Yield, 85%; mp. 166–168 °C; white amorphous solid.
IR (KBr) cm-1: 3409, 3329, 3206, (N-H, str, symm. & asymm.), 3062 (CH), 1714 (C=O, amide I ), 1629 (N-H, amide II), 1536 (C=C), 1078, 1052, 983, 837.
1H-NMR (300 MHz, CDCl3): δ 2.35 (t, 2H, CH2), 2.87 (t, 2H, CH2), 7.27–7.92 (m, 7H, Ar-H, NH2, partially D2O-exchangeable), 9.78 (s, 1H, CONH, D2O-exchangeable).
13C-NMR (75 MHz, DMSO-d6) δ; 29.4 (CH2), 36.1 (CH2), 127.67 (CH), 128.3 (CH), 129.1 (CH), 130.6 (C), 149.2 (C=N), 169.8 (N-CO-N), 178.3 (C-CO-N).
Anal. Calcd for C11H12N4O2: C, 56.89; H, 5.21; N, 24.12. Found: C, 56.68; H, 5.26; N, 24.31. MS-(ESI); m/z 233 (M+1).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Demirayak, S.; Karaburun, A.C.; Beis, R. Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur. J. Med. Chem. 2004, 39, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, M.; Utku, S.; Küpeli, E. Synthesis and analgesic and anti-inflammatory activities 6-substituted-3(2H)-pyridazinone-2-acetyl-2-(p-substituted/nonsubstitutedbenzal)hydrazine derivatives. Eur. J. Med. Chem. 2009, 44, 3760–3764. [Google Scholar] [CrossRef] [PubMed]
- Rathish, I.G.; Javed, K.; Bano, S.; Ahmad, S.; Alam, M.S.; Pillai, K.K. Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives. Eur. J. Med. Chem. 2009, 44, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Radeke, H.; Azure, M.; Hanson, K.; Benetti, R.; Su, F.; Yalamanchili, P.; Yu, M.; Hayes, M.; Guaraldi, M.; Kagan, M.; Robinson, S.; Casebier, D. Synthesis and biological evaluation of pyridazinone analogues as potential cardiac positron emission tomography tracers. J. Med. Chem. 2008, 51, 2954–2970. [Google Scholar]
- Sönmez, M.; Berber, I.; Akbaş, E. Synthesis, antibacterial and antifungal activity of some new pyridazinonemetalcomplexes. Eur. J. Med. Chem. 2006, 41, 101–2105. [Google Scholar] [PubMed]
- Siddiqui, A.A.; Ahamad, S.R.; Mir, M.S.; Hussain, S.A.; Raish, M.; Kaur, R. Synthesis and in vitro antifungal activity of 6-substituted-phenyl-2-{[(4’-substituted phenyl-5’-thioxo)-1,2,4-triazol-3-yl]-methyl}-2,3,4,5-tetrahydropyridazin-3-one derivatives. Acta Poloniae Pharm. 2008, 65, 223–228. [Google Scholar] [PubMed]
- Khan, M.S.Y.; Siddiqui, A.A. Pyridazinone derivatives: A potent anti-inflammatory agents. Indian J. Chem. 2000, 39B, 614. [Google Scholar]
- Sample Availability: Sample of the compound 2 is available from authors.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).