Abstract
The title compound, 2,6-bis(ethyl-9-ethyl-9H-carbazolylmethylene)cyclohexanone has been synthesized by condensation of 9-ethylcarbazole-3-aldehyde and cyclohexanone in ethanol in the presence of pyridine. The structure of this new compound was confirmed by elemental analysis, IR, 1H NMR, 13C NMR and EI-MS spectral analysis.
Knoevenagel condensations are among the frequently applied reactions in organic synthesis, providing a good synthetic pathway to form donor-acceptor dienes known as methylene [1]. Compounds with donor-acceptor dienes have become of much interest in recent years in the context of photoelectronics [2], photophotonics [3], photodynamic therapy [4], electrochemical sensing [5], optical limiting [6], langmuir film and photoinitiated polymerization [7]. As evident from the literature, it was noted that a lot of research has been carried out on donor-acceptor dienes, but no work has been done on bis-donor-acceptor dienes. In this paper we report the synthesis of a bis-carbazole from a carbazole aldehyde. With regard to the stereochemistry of the product, we assume the E,E-isomer (only one singulet of the olefinic protons was observed in the 1H NMR spectrum) for steric reasons, but the Z,Z-isomer cannot be completely excluded.
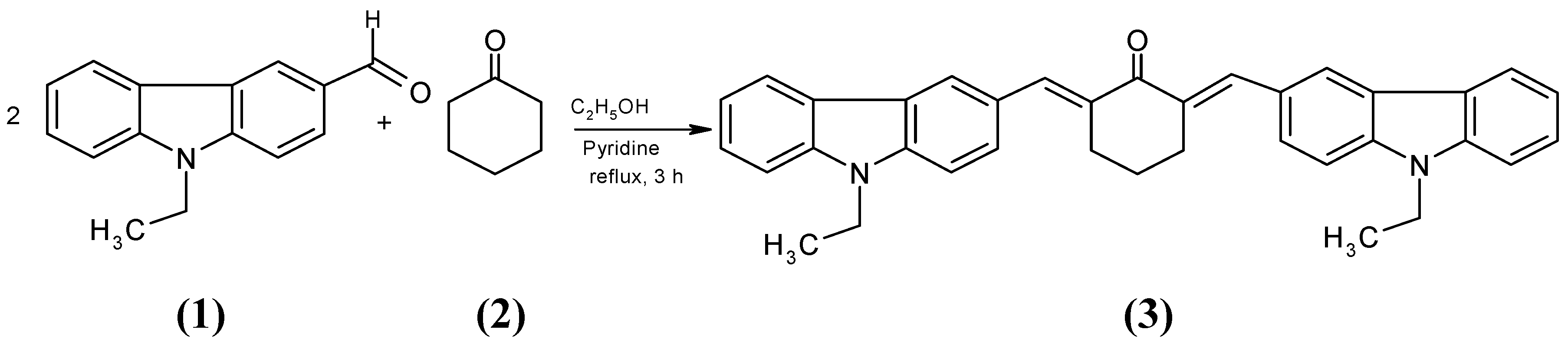
A mixture of 9-ethylcarbazole-3-aldehyde (1.0 g, 0.0044 mol), cyclohexanone (0.215 g, 0.0022 mol) and a few drops of pyridine in ethanol (15 mL) was heated for 3 h. The progress of the reaction was monitored by TLC. The solid that separated from the cooled mixture was collected and recrystallized from a methanol-chloroform mixture.
Yield: 20%; m.p. 81 °C.
EI-MS m/z (rel. int.%): 509 (50) [M+1]+.
IR (KBr) vmax cm-1: 3053 (C-Haromatic), 1681 (C=O), 1589 (C=C), 1147 (C-N).
1H NMR (600 MHz, CDCl3) δ: 8.17 (H-1, d, J = 7.8 Hz), 8.02 (H-2, d, J = 8.4 Hz), 7.25 (H-3, s), 7.55 (H-4, d, J = 7.2 Hz), 7.49 (H-5, dd, J = 8.4 Hz, 7.8 Hz), 7.34 (H-6, dd, J = 7.2 Hz, 1.2 Hz), 7.55 (H-7, dd, J = 7.2 Hz, 7.8 Hz), 8.65 (H-8, s), 4.43 (CH3-CH2-N, t, J = 7.2 Hz), 1.64 (CH3-CH2-N, q, J = 7.2 Hz).
13C NMR (600 MHz, CDCl3) δ: 201.74, 198.65, 191.89, 145.15, 143.55, 140.64, 140.33, 139.96, 137.96, 129.06, 128.43, 127.20, 126.72, 126.11, 125.88, 125.11, 124.37, 123.03, 122.84, 121.53, 120.98, 120.30, 119.68, 109.31, 108.94, 108.33, 58.49, 40.17, 37.93, 29.24, 27.35, 23.95, 13.84.
Anal. calc. for C36H32ON2: C, 85.03, H, 6.29, N, 5.51. Found: C, 84.98, H, 6.24, N, 5.47.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the Chemistry Department, King Abdul Aziz University, Jeddah, Saudi Arabia for providing the research facilities and the deanship of scientific research for the financial support of this work via grant No. 30-50/429.
References and Notes
- Asiri, A.M. Synthesis and characterization of dyes exemplified by 2-arylidene-1-dicyanomethyleneindane. Dyes Pigm. 1999, 42, 209–213. [Google Scholar] [CrossRef]
- Rosso, V.; Loicp, J.; Renotte, Y.; Lion, Y. Optical non-linearity in Disperse Red 1 dye-doped sol gel. J. Non-Cryst. Solids 2004, 342, 140–145. [Google Scholar] [CrossRef]
- Cotter, D.; Manning, R.J.; Blow, K.J.; Ellis, A.D.; Kelly, A.E.; Nesset, D. Nonlinear optics for high-speed digital information processing. Science 1999, 286, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, P. On the optical limiting and Z-scan of hexamethylin-dotricarbocyanine perchlorate dye. Opt. Mater. 2009, 31, 1559–1563. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Giglio, A.D.; Oliveir, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; Turchiello, R.F.; Baptist, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Tonle, I.K.; Ngameni, E.; Tcheumi, H.L.; Tchieda, V.; Carteret, C.; Walcarius, A. Sorption of methylene blue on an organoclay bearing thiol groups and application to electrochemical sensing of the dye. Talanta 2008, 74, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Fernandez, A.L.; Munoz, E.; Martin, M.T.; Camacho, L. Langmuir-Blodgett films containing water-soluble molecules: The methylene blue-dimyristoyl phosphatidic acid system. Thin Solid Films 2008, 284–285, 162–165. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).