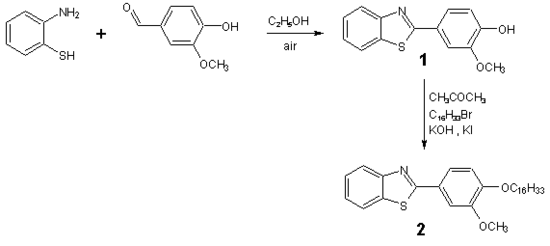
2-[4-(Hexadecyloxy)-3-methoxyphenyl]-1,3-benzothiazole
Abstract
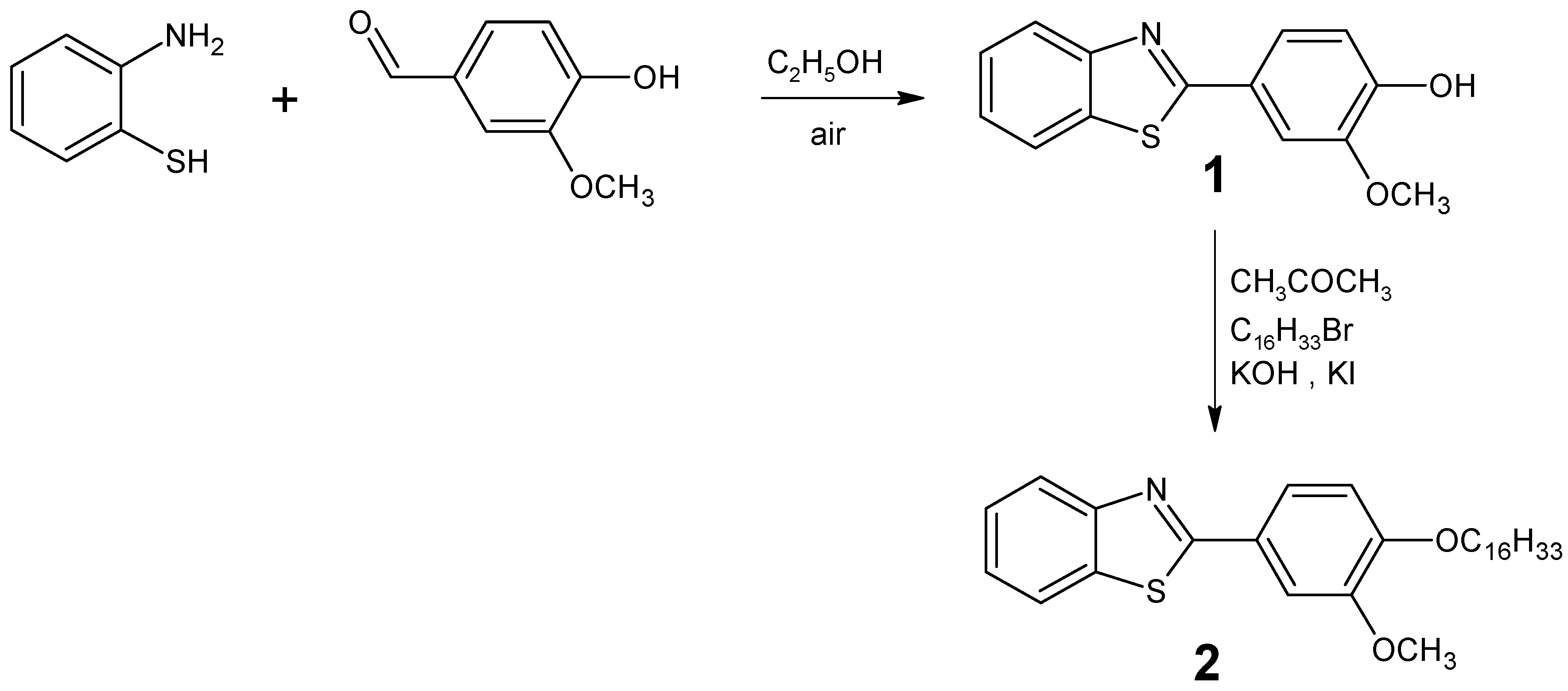
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Lai, C.K.; Liu, H.C.; Li, F.J.; Cheng, K.L.; Sheu, H.S. Heterocyclic benzoxazole-based liquid crystals. Liq. Cryst. 2005, 32, 85–94. [Google Scholar] [CrossRef]
- Meyer, E.; Zucco, C.; Gallardo, H. Metallomesogens: Synthesis and properties. J. Mater. Chem. 1998, 8, 1351–1354. [Google Scholar] [CrossRef]
- Ha, S.T.; Koh, T.M.; Yeap, G.Y.; Lin, H.C.; Boey, P.L.; Yip, F.W.; Ong, S.T.; Ong, L.K. Synthesis and mesomorphic properties of 2-(4-alkyloxyphenyl)benzothiazoles. Mol. Cryst. Liq. Cryst. 2009, 506, 56–70. [Google Scholar] [CrossRef]
- Ha, S.T.; Koh, T.M.; Ong, S.T.; Lee, T.L.; Sivasothy, Y. Synthesis of 2-(4-propyloxyphenyl)benzothiazole. Molbank 2009, 3, M609. [Google Scholar] [CrossRef]
- Ha, S.T.; Koh, T.M.; Ong, S.T.; Ong, L.K. Synthesis of a new heterocycle with liquid crystal properties: 2-(3-Methoxy-4-hexadecanoyloxyphenyl)benzothiazole. Molbank 2009, 2009, M606. [Google Scholar] [CrossRef]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals; Taylor & Francis Ltd.: London, UK, 1998. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ha, S.-T.; Koh, T.-M.; Ong, S.-T.; Win, Y.-F. 2-[4-(Hexadecyloxy)-3-methoxyphenyl]-1,3-benzothiazole. Molbank 2009, 2009, M634. https://doi.org/10.3390/M634
Ha S-T, Koh T-M, Ong S-T, Win Y-F. 2-[4-(Hexadecyloxy)-3-methoxyphenyl]-1,3-benzothiazole. Molbank. 2009; 2009(4):M634. https://doi.org/10.3390/M634
Chicago/Turabian StyleHa, Sie-Tiong, Teck-Ming Koh, Siew-Teng Ong, and Yip-Foo Win. 2009. "2-[4-(Hexadecyloxy)-3-methoxyphenyl]-1,3-benzothiazole" Molbank 2009, no. 4: M634. https://doi.org/10.3390/M634
APA StyleHa, S.-T., Koh, T.-M., Ong, S.-T., & Win, Y.-F. (2009). 2-[4-(Hexadecyloxy)-3-methoxyphenyl]-1,3-benzothiazole. Molbank, 2009(4), M634. https://doi.org/10.3390/M634