Introduction
A calixarene is a macrocycle or cyclic oligomer based on a condensation product of a phenol and an aldehyde [1]. The word calixarene is derived from calix or chalice because this type of molecule resembles a vase and from the word arene that refers to the aromatic building blocks. Calixarenes have hydrophobic cavities that can hold smaller molecules or ions and belong to the class of cavitands known in Host-Guest chemistry. Calixarene nomenclature is straightforward and involves counting the number of repeating units in the ring and include it in the name. A calix[4]arene has four units in the ring and a calix[6]arene has six.
Experimental
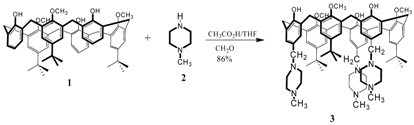
CH2O (0.189 g, 6.372 mmol) and 1-methyl piperazine (2) (0.370 g, 4.25 mmol) was added to solution of tri-tert-butyl trimethoxy calix[6]arene (1) [2] (0.300 g, 0.35 mmol) in THF (10 ml) and acetic acid (10 ml). The mixture was stirred for 64 h at room temperature. The solvents were removed under reduced pressure and water (20 ml) was added. Afterwards, a 10% solution of K2CO3 in water (40ml) was added. The product 3 precipitated (0.242 g, 86 %).
Mp 158-160 °C.
1H NMR (200 MHz, CDCl3): δH 6.99 (s, 6H+3H, ArH+ArOH); 6.89 (s, 6H, ArH); 3.88 (s, 12H, ArCH2Ar); 3.54 (s, 9H, ArOCH3); 3.38 (s, 6H, ArCH2N); 2.43 (s large, 24H, NCH2CH2NCH2CH2); 2.27 (s, 9H, NCH3), 1.03 (s, 27H, C(CH3)3).
Anal Found: C, 75.89; H, 8.31%. Calc. for C75H102O6N6: C, 76.10; H, 8.69%. FAB(+)MS: m/z = 1183.8 (M)+, 1182.8 (M–H)+.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Gutsche, C.D. Calixarenes; Royal Society of Chemistry: Cambridge, 1989. [Google Scholar]
- Casnati, A.; Domiano, L.; Pochini, A.; Ungaro, R.; Carramolino, M.; Oriol-Magrans, J.; Nieto, P.M.; Lopez-Prados, J.; Prados, P.; de Mendoza, J.; Janssen, R. G.; Verboom, W.; Reinhoudt, D.N. Tetrahedron 1995, 51, 12699–12720.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).