Synthesis of 5,17,29- tri[(4-methyl-1-piperazinyl)methyl]- 11,23,35-tri-tert-butyl-37,39,41-trimethoxy-calix[6]arene
Introduction
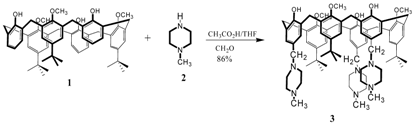
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Gutsche, C.D. Calixarenes; Royal Society of Chemistry: Cambridge, 1989. [Google Scholar]
- Casnati, A.; Domiano, L.; Pochini, A.; Ungaro, R.; Carramolino, M.; Oriol-Magrans, J.; Nieto, P.M.; Lopez-Prados, J.; Prados, P.; de Mendoza, J.; Janssen, R. G.; Verboom, W.; Reinhoudt, D.N. Tetrahedron 1995, 51, 12699–12720.
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Souane, R.; Vicens, J. Synthesis of 5,17,29- tri[(4-methyl-1-piperazinyl)methyl]- 11,23,35-tri-tert-butyl-37,39,41-trimethoxy-calix[6]arene. Molbank 2008, 2008, M575. https://doi.org/10.3390/M575
Souane R, Vicens J. Synthesis of 5,17,29- tri[(4-methyl-1-piperazinyl)methyl]- 11,23,35-tri-tert-butyl-37,39,41-trimethoxy-calix[6]arene. Molbank. 2008; 2008(3):M575. https://doi.org/10.3390/M575
Chicago/Turabian StyleSouane, Rachid, and Jacques Vicens. 2008. "Synthesis of 5,17,29- tri[(4-methyl-1-piperazinyl)methyl]- 11,23,35-tri-tert-butyl-37,39,41-trimethoxy-calix[6]arene" Molbank 2008, no. 3: M575. https://doi.org/10.3390/M575
APA StyleSouane, R., & Vicens, J. (2008). Synthesis of 5,17,29- tri[(4-methyl-1-piperazinyl)methyl]- 11,23,35-tri-tert-butyl-37,39,41-trimethoxy-calix[6]arene. Molbank, 2008(3), M575. https://doi.org/10.3390/M575



