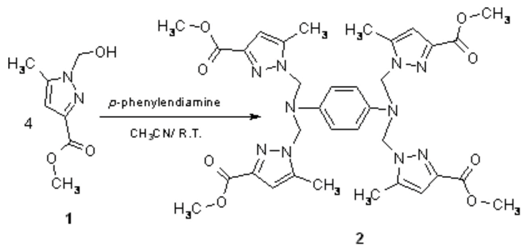
The product 2 was prepared by the addition of p-phenylendiamine (NH2C6H4NH2) to 1 [1] according to the reported procedure [2]. To a solution of 1 (3.4 g, 20 mmol) in acetonitrile (45 ml) was added p-phenylendiamine (0.54 g, 5 mmol) and the mixture was stirred. The stirring was continued at room temperature for a week. The residue was precipitated by addition of cold water, purified and dried by hexane and under vacuum, to afford 2.63 g (74% yield) 2 as a white solid.
Melting Point: 197 °C.
IR (KBr, cm–1): 3140 (=CH); 2990 (CH); 1730 (C=O); 1540 (C=C); 1480 (-C=N); 1450; 1410.
1H-NMR (400 MHz, CDCl3): δ= 7.25 (s, 4H, Ph); 6.5 (s, 4H, Pyrazol); 5.6 (s, 8H, N-CH2-N); 4 (s, 12H, O-CH3); 2.2 (s, 12H, CH3).
MS (m/z): 739 [M + Na+]; 716 [M]+; 613; 585; 460.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
We are indebted to the "Programme Thématique d'Appui à la Recherche Scientifique PROTARS N° P1T2/27", and the "Wylaya d'Oujda" for financial support.
References
- Dvoretzky, I.; Richter, G.H. J.Org.Chem. 1950, 15, 1285. [CrossRef]
- Sheu, S-C.; Tien, M-J.; Cheng, M-C.; Ho, T-I.; Peng, S-M.; Lin, Y-C. J. Chem. Soc. Dalton Trans. 1995, 3503–3510.
- Sample Availability: Available from the authors and MDPI.
© 2005 MDPI. All rights reserved.