Despite the numerous polydentate macrocyclic structures already described as neutral receptors for various substrates [1,2,3], the search for new receptor families is still important with a view to obtaining molecules capable of molecular recognition, transport, selective catalysis or biological models.
In this work, we were interested in mixed donor macrocycle incorporating sp3 and sp2 nitrogens; some receptors of this type have been described to the cyclam family and are obtained from 2,6 disubstituted pyridines [4,5].
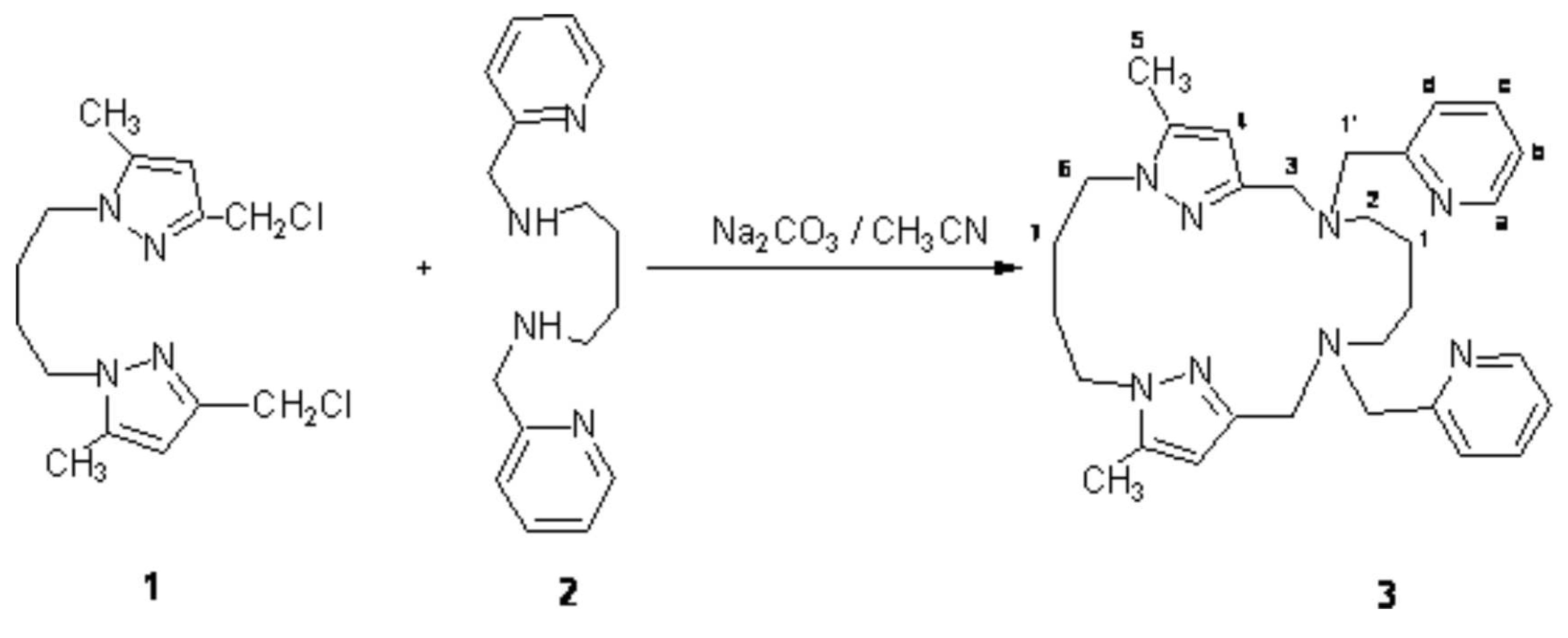
A suspension of sodium carbonate (12 g, 120 mmol) in acetonitrile (250 mL) was refluxed under magnetic stirring, then a solution of 1,4-bis(3-chloromethyl-5-methylpyrazolyl)butane 1 (2.1 g, 7 mmol) [1] and N,N'-bis(pyridin-2-ylmethyl)propane-1,3-diamine 2 (1.8 g , 7 mmol) [6] in acetonitrile (50 mL) was added dropwise. The solution was refluxed under stirring for two hours, filtered and the solvent was removed in vacuum, the residue was purified on alumina column with (CH2Cl2/MeOH: 95/5) as eluent to give the 2.70 g (90% yield) macrocycle 3 as an oily substance.
1H-NMR (250 MHz; CDCl3): δ= 8.50 Ha; 7.65 Hc; 7.57 Hd; 7.10 Hb; 6.00 (s, 2H, H4); 4.00 (t, 4H, H6); 3.82 (s, 4H, H3); 3.55 (s, 4H, H1'); 2.33 (t, 4H, H2); 2.24 (d, 6H, H5); 1.38 (m, 2H, H1); 1.75 (m, 4H, H7).
MS (FAB; m/z): 513 [M+H]+.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Izatt, R. M.; Christensen, J. J. Progress in Macrocyclic Chemistry; Wiley-Interscience: New York, 1979; Vol. 11981; Vol. 2.
- Melson, G. A. Coordination Chemistry of Macrocycles; Plenum: New York, 1979. [Google Scholar]
- Bol, J. E.; Driessen, W. L.; Reedijk, J. J. Chem. Soc. Chem. Comm. 1995, 1365.
- Lindoy, L. F. The Chemistry of Macrocyclic Ligand Complexes. Cambridge University Press: Cambridge, 1988. [Google Scholar]
- Hancok, R. D.; Martell, A. E . Chem. Rev. 1989, 89, 1875.
- Hartman, J. A. R.; Kammier, A. L.; Spracklin, R. J.; Pearson, W. H.; Combariza, M. Y.; Vachet, R. W. Inorganica Chimica Acta 2004, 357, 1141.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.