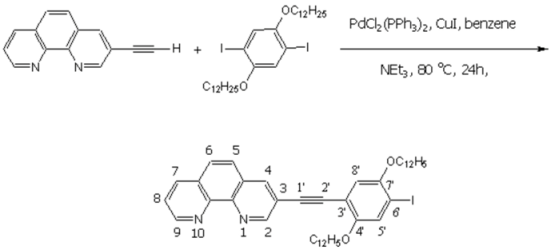
The experimental procedure follows a protocol developed by Sonogashira [1]. All reactions were carried out under the atmosphere of dry argon by using standard Schlenk tube techniques.
To a mixture of 3-ethynyl-[1,10]-phenanthroline [2,3] (306 mg, 1.5 mmol) and 1,4-bis-dodecyloxy-2,5-diiodobenzene [4,5,6] (5.0 g, 7.5 mmol) in dry benzene (25 mL), and triethyl amine (10 mL), were added CuI (28.6 mg, 0.15 mmol), and [PdCl2(PPh3)2] (52.6 mg, 0.075 mmol). The reaction mixture was kept at 80 oC for 24h while stirring vigorously and monitored with mass spectrometer to see the formation of he desired product. After removal of the solvent, the residue was washed with aqueous potassium cyanide (2%, 30 mL) and distilled water (100 mL), and purified by column chromatography (SiO2, CHCl3) to collect 3-(2,5-bis-dodecyloxy-4-iodo-phenylethynyl)-[1,10]-phenanthroline as a fatty solid (232.45 mg, 0.3 mmol, 20%).
Melting point: > 300 °C.
IR (KBr, cm-1): 3205, 2202, 1590, 1477, 1415, 1261, 1202, 1095, 1053, 940, 818, 729.
1H-NMR (250 MHz, d6-acetone): δ=0.95 (6H, 2CH3); 1.3 (36H, 18CH2); 1.7 (4H, 2CH2); 4.0 (4H, 2CH2); 6.6 (1H, 8'-H); 7.0 (1H, 5'-H); 7.25 (1H, 8-H); 7.4 (1H, 5-H); 7.7 (1H, 6-H); 8.0 (1H, 7-H); 8.2 (1H, 4-H); 8.8 (1H, 9-H); 9.0 (1-H, 2-H).
Elemental Analysis: Calculated for C44H59IN2O2: C, 68.2%; H, 7.67%; N, 3.62%. Found: C, 68%; H, 7.5%; N, 3.80.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The author gratefully acknowledges the financial supports from the Bu Ali Sina University, Hamedan, Iran.
References
- Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975, 4467.
- Michel, C.; Habibi, D.; Schmittel, M. Molecules 2001, M224.
- Michel, C.; Habibi, D.; Schmittel, M. Molecules 2001, M225.
- Giesa, R.; Schulz, R. C. Makromol. Chem. 1990, 191, 857.
- Weder, C.; Wrighton, M. S. Macromolecules 1996, 29, 5157.
- Swager, T. M.; Gil, C. J.; Wrighton, M. S. J. Phys. Chem 1995, 99, 4886.
© 2005 MDPI. All rights reserved.