There is considerable interest in the synthesis of new materials with large second-order optical non-linearity because of their potential applications in optical data storage, telecommunications and optical signals processing [1,2,3,4]. First and second hyperpolarizability can be observed with molecules which possess an electron donor and acceptor groups connected by a conjugated Π-electron bridge which allow the possibility of intramolecular charge transfer [5,6].
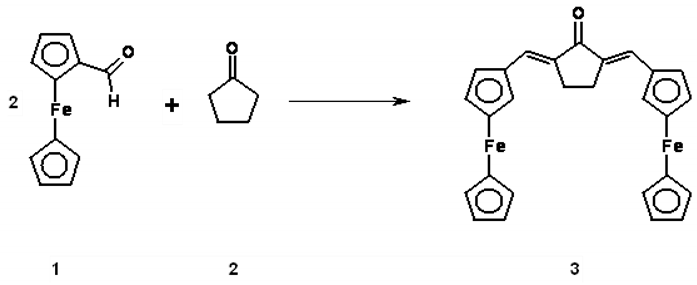
To a well stirred solution of (Fc-CHO) (2.5g, 11.7 mmole) cyclopentanone (0.98 g, 15.8 mmole) in ethanol (30 mL) was added dropwise a solution of NaOH (30 mL, 10%) at 60°C over a period of 20 minutes. After the addition was completed the solution was left to stir at room temperature for 12 hours, and then poured into ice-cold water (200 ml) and stir for 2 hours. The precipitated chaconne was filtered off and washed with copious amount of water until the wash was neutral, then washed with cold ethanol and dried. The solid products were recrystallized from ethanol to give yellow crystals (5.2 g, 93.4%).
Melting Point: 200-202 °C (uncorrected).
UV (EtOH, λmax nm; ε dm3.mol-1.cm-1): 350 (46692); 525 (12356).
IR (KBr; cm-1): 1690 (C=O); 1625, 1610 (C=C); 1110, 990, 813.
1H-NMR (400 MHz; CDCl3): δ= 7.44 (s, 2H, -CH=C); 4.60 (broad s, 4H, H-2, H-5); 4.51 (broad s, 4H, H-3, H-4); 4.17 (s, 10H, C5H5); 2.81 (broad s, 4H, CH2).
Elemental Analysis: Calculated for C27H24OFe2 (476.27): C, 68.13%; H, 5.04%. Found: 68.25%; H, 4.91%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Boyd, R.W. Nonlinear Optics; Academic Press: New York, 1992. [Google Scholar]
- Marder, S.R.; Beratan, D.N.; Cheng, L.T. Science 1991, 252, 103.
- Eaton, D.F. Science 1991, 253, 281.
- Staring, E.G. Recl. Trav. Chim. Pays-Bas 1991, 110, 492.
- Derik, C.W.; Wagniere, R.J. J. Am. Chem. Soc. 1986, 108, 5387.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.