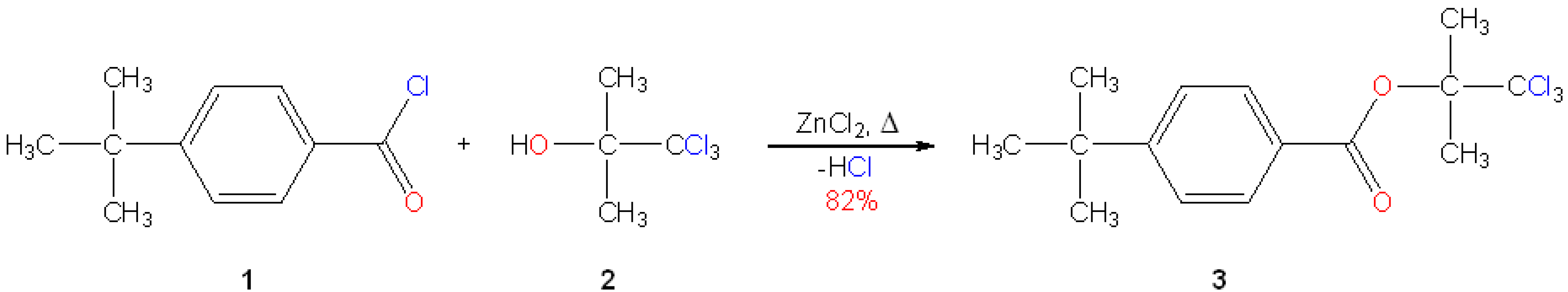
In our previous work we have demonstrated the utility of the 2,2,2-trichloro-1,1-dimethylethyl (b,b,b-trichloro-tert-butyl, Tcb) group for the protection of carboxylic acids [1]. Herein we would like to describe the preparation of one of their representatives, the title aromatic ester. The procedure applied by us is based on the work by Gupta and Srivastava [2].
Experimental: Freshly prepared anhydrous zinc chloride (0.41 g, 3.0 mmol) was chilled in an ice-bath (0-5 °C), then 4-tert-butylbenzoyl chloride (2.73 mL, 2.95 g, 15.0 mmol) and anhydrous b,b,b-trichloro-tert-butanol (1.775 g, 10.0 mmol, prepared by in vacuo drying of the commercial hemihydrate over phosphorus pentoxide) were added, each in one portion. The flask was removed from the ice-bath and gently shaken to initiate the evolution of hydrogen chloride. When the gas evolution had subsided the reaction mixture was heated at 100 °C for 2 h. The mixture was diluted with diethyl ether (100 mL), washed with cold satd. NaHCO3 solution (3 x 50 mL) and brine (50 mL), respectively. The organic phase was dried (MgSO4) and evaporated. The oily residue solidified upon standing (2.756 g, 82%, TLC: single spot). An analytical sample was obtained by recrystallization from methanol (14 mL).
Mp. 78.7-79.8 °C.
TLC: light petroleum-ethyl acetate 7:3, Rf: 0.76.
Anal. calcd. for C15H19Cl3O2 (337.669): C, 53.35; H, 5.67; Cl, 31.50%; found C, 53.48; H, 5.78; Cl, 31.29%.
IR (ν, cm-1, CHCl3, KBr): 705m, 789m, 806m, 856w, 1016w, 1119s, 1193m, 1284s, 1370w, 1387w, 1410w, 1462w, 1606w, 1722s (νC=O), 2871w, 2905w, 2967m.
1H NMR (CDCl3, 500 MHz, ppm): 1.34 (s, 9H, tert-Bu), 2.03 (s, 6H, (CH3)2C), 7.48 (d, 2H, J=8.5 Hz, arom. CHs), 8.00 (d, 2H, J=8.5 Hz, arom. CHs).
13C NMR (CDCl3, 125 MHz, ppm, assignment based on HMBC and HMQC experiments): 21.42 ((CH3)2CCCl3), 31.09 ((CH3)3C), 35.11 ((CH3)3C), 88.97 (CCl3), 107.12 ((CH3)2CCCl3), 125.47 (arom. CHs), 127.89 (arom. quaternary C), 129.74 (arom. CHs), 156.99 (arom. quaternary C), 164.46 (COO).
CI-MS (isobutane, m/z): 341, 339, 337 ([M+H]+, isotopic peaks); 305, 303 ([M-Cl]+, isotopic peaks). The calculated peak distributions correspond to the expected structure.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Kovács, L.; Forgó, P.; Kele, Z. Fourth International Electronic Conference on Synthetic Organic Chemistry. abstr. no. a0076 (http://www.unibas.ch/mdpi/ecsoc-4/a0076/a0076.htm).
- Gupta, I.; Srivastava, N.P. Recl. Trav. Chim. Pays-Bas 1956, 75, 48–50.
- Sample availability: sample available from the authors.
© 2004 MDPI. All rights reserved.