To continue our investigation into the chemistry of furan derivatives [1, 2], we have synthesized furylphenylketone bearing nitro-group in the ortho-position of benzene ring. We developed reaction conditions for preparation of this compound smoothly and in rather high yield.
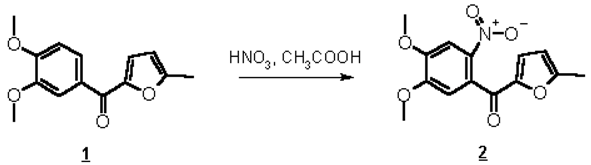
To an ice-cooled solution of ketone 1 (1.00 g, 4 mmol) in glacial acetic acid (10 mL) red fuming nitric acid (1 mL) was added dropwise. The reaction mixture was kept at 0 °C for 10-15 min (TLC monitoring) and then poured into water. The precipitate obtained was filtered off and air-dried. Recrystallization from ethanol with charcoal afforded nitroketone 2 as yellow crystals (0.76 g, 65 %).
Mp: 147-148 °C (ethanol).
IR (KBr): 1695, 1640 cm-1.
1H NMR (CDCl3, 60 MHz, ppm): 2.35 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 4.03 (s, 3H, OCH3), 6.14 (d, 1H, J = 3.2, 3-HFur), 6.95 (d, J = 3.2, 4-HFur), 6.98 (s, 1H, HAr), 7.68 (s, 1H, HAr).
Anal. calc. for C14H13NO6: C 57.73, H 4.50, N 4.81; Found: C 57.79, H 4.42, N 4.77.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References:
- Butin, A.V.; Stroganova, T.A.; Lodina, I.V.; Krapivin, G.D. Tetrahedron Letters 2001, 42, 2031.
- Butin, A.V.; Abaev, V.T.; Stroganova, T.A.; Gutnov, A.V. Molecules 1997, 2, 62.
© 2003 MDPI. All rights reserved.