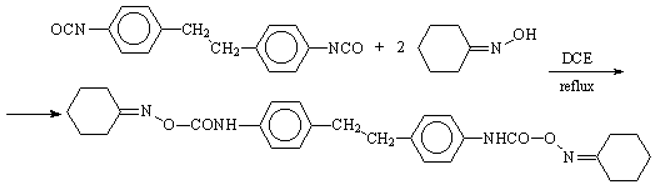
The experimental procedure follows the general synthesis of blocked isocyanates [1,2]. Thus, a mixture of 1,2-bis(p-isocyanato-phenyl)ethane (4,4'-dibenzyl diisocyanate) [3,4] (66 g, 0.25 mol) and cyclohexanone oxime (65 g, 0.57 mol) was dissolved in 450 mL 1,2-dichloroethane and refluxed while stirring for 2-4 h. The product of reaction precipitated with the reaction progressed. The progress of the reaction was followed by IR spectroscopy. After cooling, the solid was isolated by filtration, washed several times with acetone and dried at 100°C. The product has a white crystalline aspect (117 g, 95.51% yield).
M.p.: Between 241-250°C a mesophase was observed followed by a decomposition process (thermo-optical method).
IR (KBr, cm-1): 3350 ms (NH), 2920 ms, 2850 m (CH2), 1730 s (CONH), 1700-1680 m (sh, C=N oxime), 1580 m (NHCO, p-Ar), 1500-1490 s (p-Ar), 1410 s (Ar, CH2-N), 1320-1310 mw (CH2), 1170 s (CH2), 1130 m (C-O, C-N), 1000 s (C-O), 910 ms (C-C, skeletal), 880 m, 860 m (C-N), 830 s (p-Ar), 740 m, 700 m (C-N).
1H NMR (60 MHz δ, DMSO-d6, 70°C): 8.97 (s, NH, 2H), 7.32, 7.22, 7.08, 6.9 (m, Ar, 8H), 2.86, 2.72 (d, CH2-Ar, 4H), 2.35, 1.7, 1.25 (m, CH2, 20H).
Elemental analysis: Calculated for: C28H34N4O4: C%= 68.55; H%= 6.99; N%= 11.42. Found: C%= 68.04; H%= 7.05; N%= 11.53.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Savostianoff, E. Inf. Chem. 1981, 119-126, 216-217, 1312.
- Kovalenko, L. G.; et al. Plast. Massy 1986, 11, 34.
- Caraculacu, A. A.; et al. Rom. Pat. 81,947, 1983.
- Caraculacu, A. A.; Caraculacu, G. J. Macromol. Sci. Chem. 1985, A22. 631.
© 2003 MDPI. All rights reserved.